Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The figure shown below is the heating curve obtained by heating 21.9 g of water from-10.0 to 140.0C. The region labeled a represents the
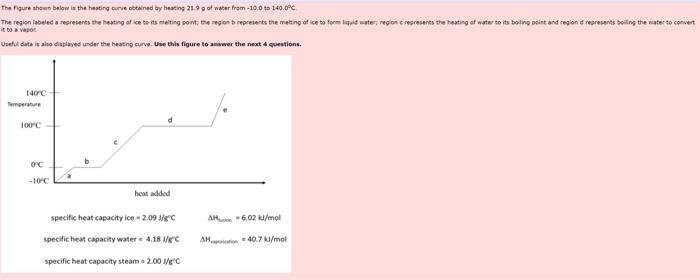
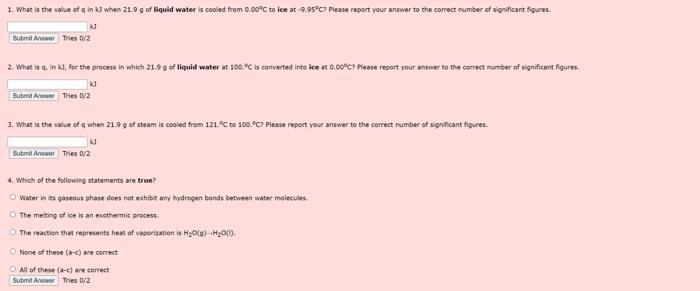
The figure shown below is the heating curve obtained by heating 21.9 g of water from-10.0 to 140.0C. The region labeled a represents the heating of ice to its melting point; the region b represents the metting of ice to form liquid water; region c represents the heating of water to its boiling point and region d represents boiling the water to convert it to a vapor Useful data is also displayed under the heating curve. Use this figure to answer the next 4 questions. 140C Temperature 100C- OFC -10C heat added specific heat capacity ice-2.09 1/gC specific heat capacity water 4.18 1/gC specific heat capacity steam = 2.00 1/g"C AH-6.02 kJ/mol AH-40.7 kJ/mol 1. What is the value of q ink when 21.9 g of liquid water is cooled from 0.00C to ice at -9.95C? Please report your answer to the correct number of significant figures. kd Submit Answer Tries 0/2 2. What is g. in k3, for the process in which 21.9 g of liquid water at 100 C is converted into ice at 0.00C? Please report your answer to the correct number of significant figures. kd Submit Answer Tries 0/2 3. What is the value of q when 21.9 g of steam is cooled from 121.C to 100.9C7 Please report your answer to the correct number of significant figures. kd Submit Anwer Tries 0/2 4. Which of the following statements are true? Water in its gaseous phase does not exhibit any hydrogen bonds between water molecules The melting of ice is an exothermic process. The reaction that represents heat of vaporization is HO(g) +HO(1) None of these (a-c) are correct All of these (a-c) are correct Submit Answer Tries 0/2
Step by Step Solution
★★★★★
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
1 2 219g ice n m 2198 1217 m MW 1gm...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


