Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Arrange the following elements in order of increasing atomic size (low to high) based on periodic trends, Give no.1 to small atomic size, and
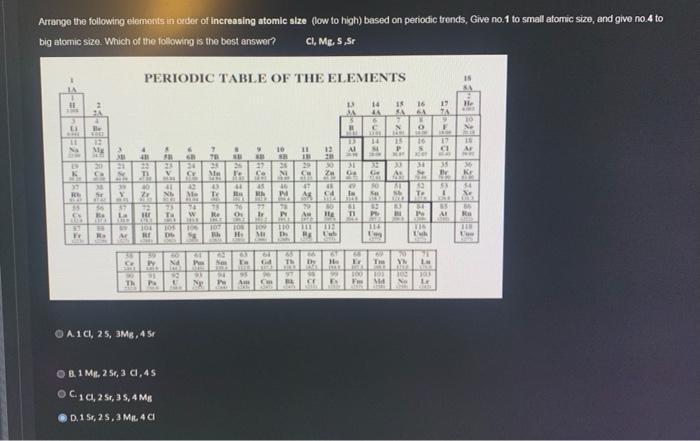
Arrange the following elements in order of increasing atomic size (low to high) based on periodic trends, Give no.1 to small atomic size, and give no.4 to big atomic size. Which of the following is the best answer? CI, Mg, S,Sr PERIODIC TABLE OF THE ELEMENTS 11 30 -3=20Det & Fr 2 4 Be VORE 32 Mg PUS 20 23 G 54 TI 45 39 38 St 95 Ba ANTA - R A 22 102 $7 La 40 M 72 HE ALE TERD 38 59 Ce Py 2003 90 Th PA 16 A1CI, 25, 3MB, 4 Sr -=95PATE B.1 Mg, 2 5r, 3 cl, 45 C10, 2 Sr, 35, 4 Mg D.1 Sr, 25, 3 Mg. 4 Cl FIL No 60 PN 6 1001 92 129 24 35 16 42 Me 2014 M THE B 192 101 105 106 107 108 109 BE Db Bh H B 7 70 11 24 Ma Xe 16 43 Te Ra Np 78 Re FM. 11 THE 16 LIBE Pas Sex Da Pr 10 6 NO 27 Se 76 23 MIN 45 RA P MBER ez zirci24 19 PD 88 95 Am On 26 le Pr Ni an 110 D 65 Th 303 BL 25+2= Dy 13 46 13 W VF 11 12 20 30 31 FISER 13 APARA l He IV MIC Ga 49 In 1144 61 TI il 100 150 RED BA F Md 18 SA 64 N AME *** 33 15 16 P 8 M 91 L O B3 IN THE SE ANDE 34 S AL 83 56 To en e I 116 Ish 20 Yh La him 103 JOLK No Le ject-01001 450 He KIME 10 *108 15 Ar NOT 36 Kr DER 54 Xe LORE 99 Ku ATE Eve
Step by Step Solution
★★★★★
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
Atomic size atomic radius decreases from left to right in a period in the periodic ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


