Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hydrogen sulfide can be removed from natural gas by the reaction H2S (9) + S02(g) = 28 (s) + 2H,0 (g) a) Calculate AH and
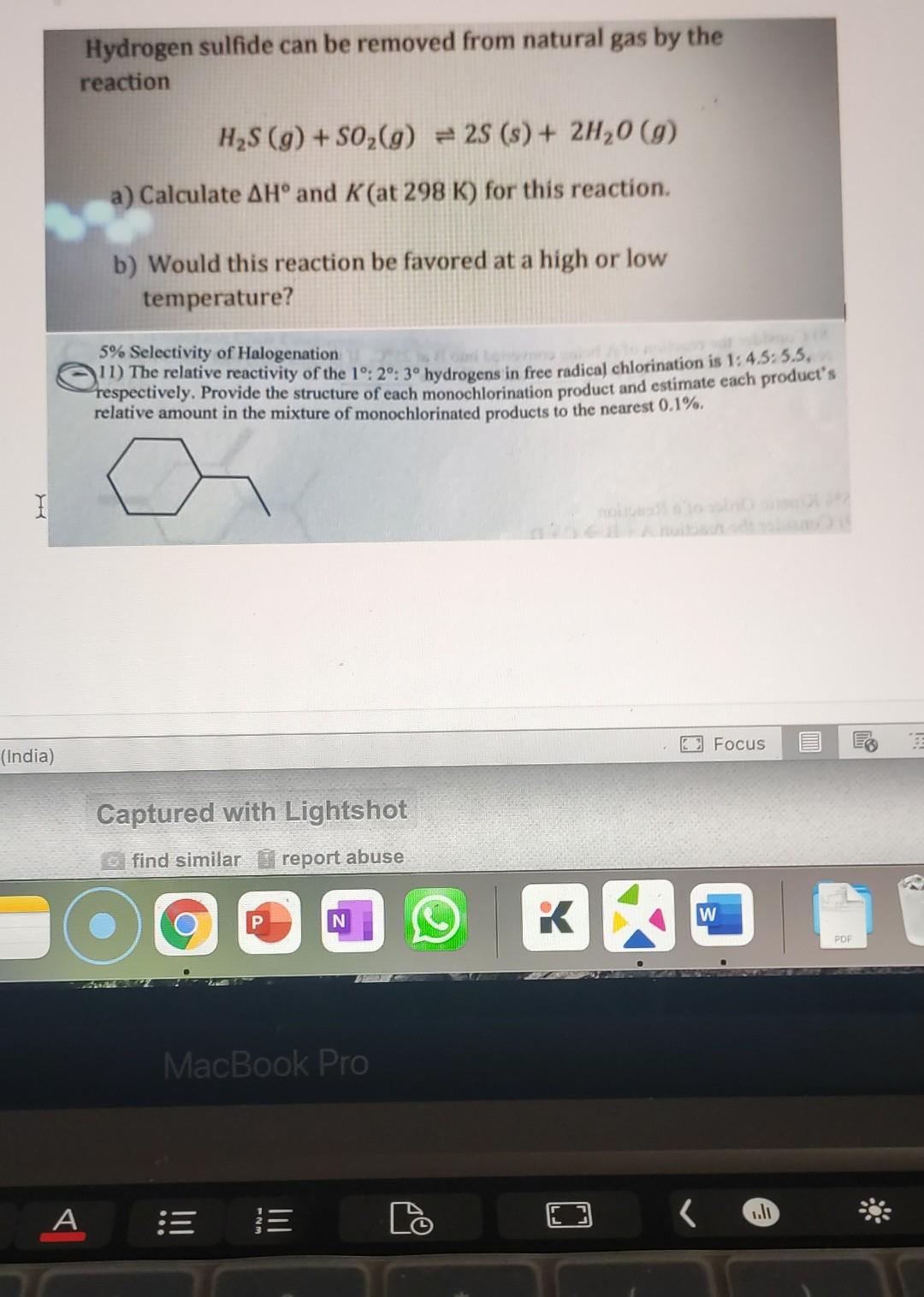
Hydrogen sulfide can be removed from natural gas by the reaction H2S (9) + S02(g) = 28 (s) + 2H,0 (g) a) Calculate AH and K (at 298 K) for this reaction. b) Would this reaction be favored at a high or low temperature? 5% Selectivity of Halogenation 11) The relative reactivity of the 10:20: 30 hydrogens in free radical chlorination is 1:4.5:55, respectively. Provide the structure of each monochlorination product and estimate each product's relative amount in the mixture of monochlorinated products to the nearest 0.1%. 2 Focus (India) Captured with Lightshot find similar report abuse O N P W RA K PDF MacBook Pro A WN- o
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


