Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hydrogenation of acetaldehyde (C3H6O) will produce 1-propanol (C3H8O). C3H6O+H2C3H8O A schematic of the reaction system is shown below. 100mol/min of propanol is produced. The fresh
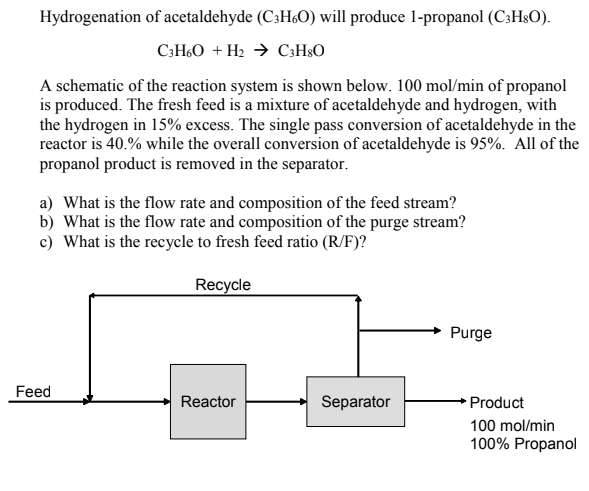 Hydrogenation of acetaldehyde (C3H6O) will produce 1-propanol (C3H8O). C3H6O+H2C3H8O A schematic of the reaction system is shown below. 100mol/min of propanol is produced. The fresh feed is a mixture of acetaldehyde and hydrogen, with the hydrogen in 15% excess. The single pass conversion of acetaldehyde in the reactor is 40.% while the overall conversion of acetaldehyde is 95%. All of the propanol product is removed in the separator. a) What is the flow rate and composition of the feed stream? b) What is the flow rate and composition of the purge stream? c) What is the recycle to fresh feed ratio (R/F)
Hydrogenation of acetaldehyde (C3H6O) will produce 1-propanol (C3H8O). C3H6O+H2C3H8O A schematic of the reaction system is shown below. 100mol/min of propanol is produced. The fresh feed is a mixture of acetaldehyde and hydrogen, with the hydrogen in 15% excess. The single pass conversion of acetaldehyde in the reactor is 40.% while the overall conversion of acetaldehyde is 95%. All of the propanol product is removed in the separator. a) What is the flow rate and composition of the feed stream? b) What is the flow rate and composition of the purge stream? c) What is the recycle to fresh feed ratio (R/F) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


