Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i absolutely cant figure this one out, please help! A hot 123.5 g lump of an unknown substance initially at 185.6 C is placed in
i absolutely cant figure this one out, please help! 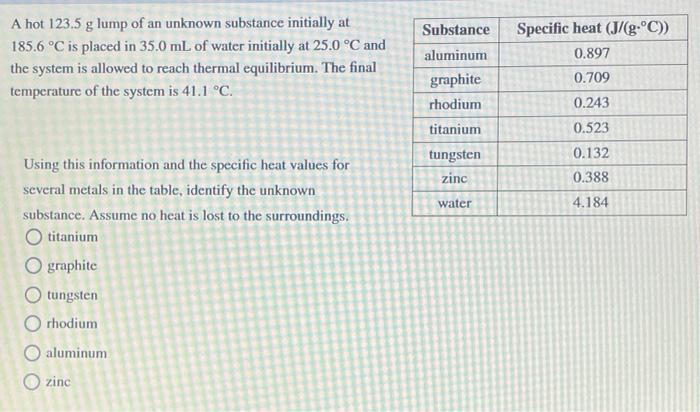
A hot 123.5 g lump of an unknown substance initially at 185.6 C is placed in 35.0 mL of water initially at 25.0 C and the system is allowed to reach thermal equilibrium. The final temperature of the system is 41.1 C. Substance aluminum graphite rhodium titanium tungsten zinc Specific heat (J/(g.C)) 0.897 0.709 0.243 0.523 0.132 0.388 water 4.184 Using this information and the specific heat values for several metals in the table, identify the unknown substance. Assume no heat is lost to the surroundings. titanium O graphite O tungsten rhodium O aluminum zinc 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


