Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I am attaching 2 Pictures the graph and the Question. Please short explanations of no more than 4-5 sentences Thanks The graph G change vs.
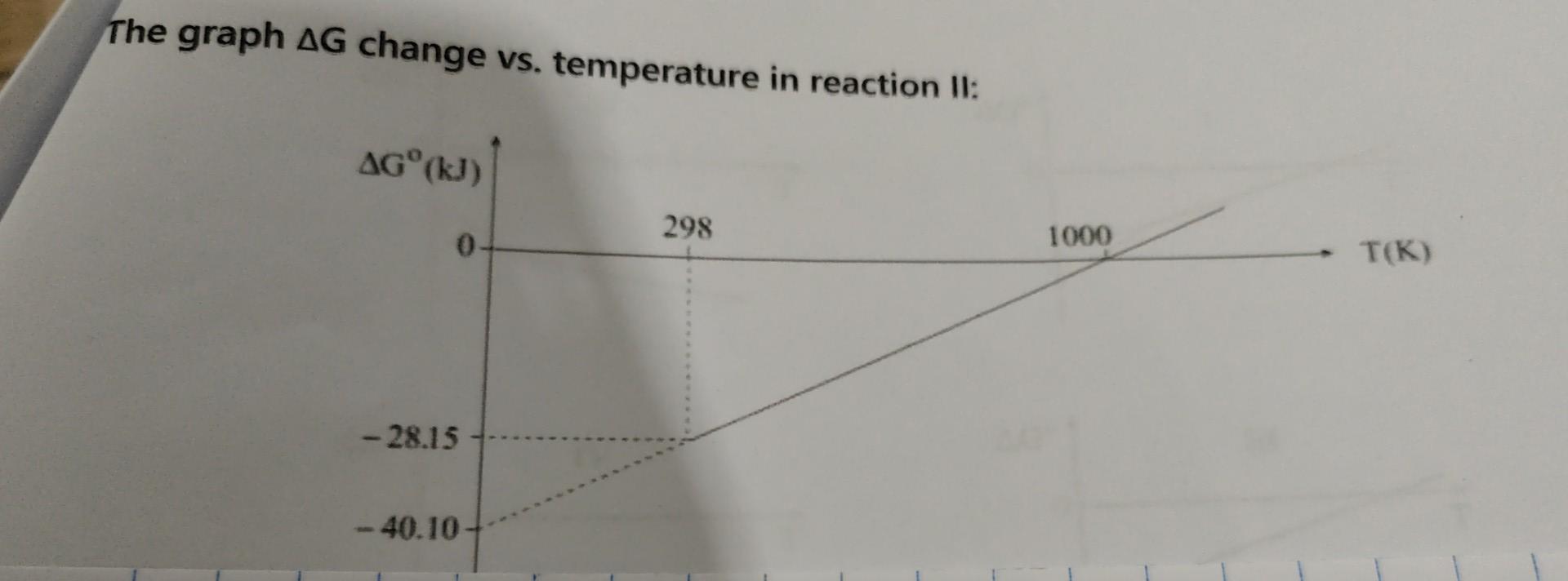
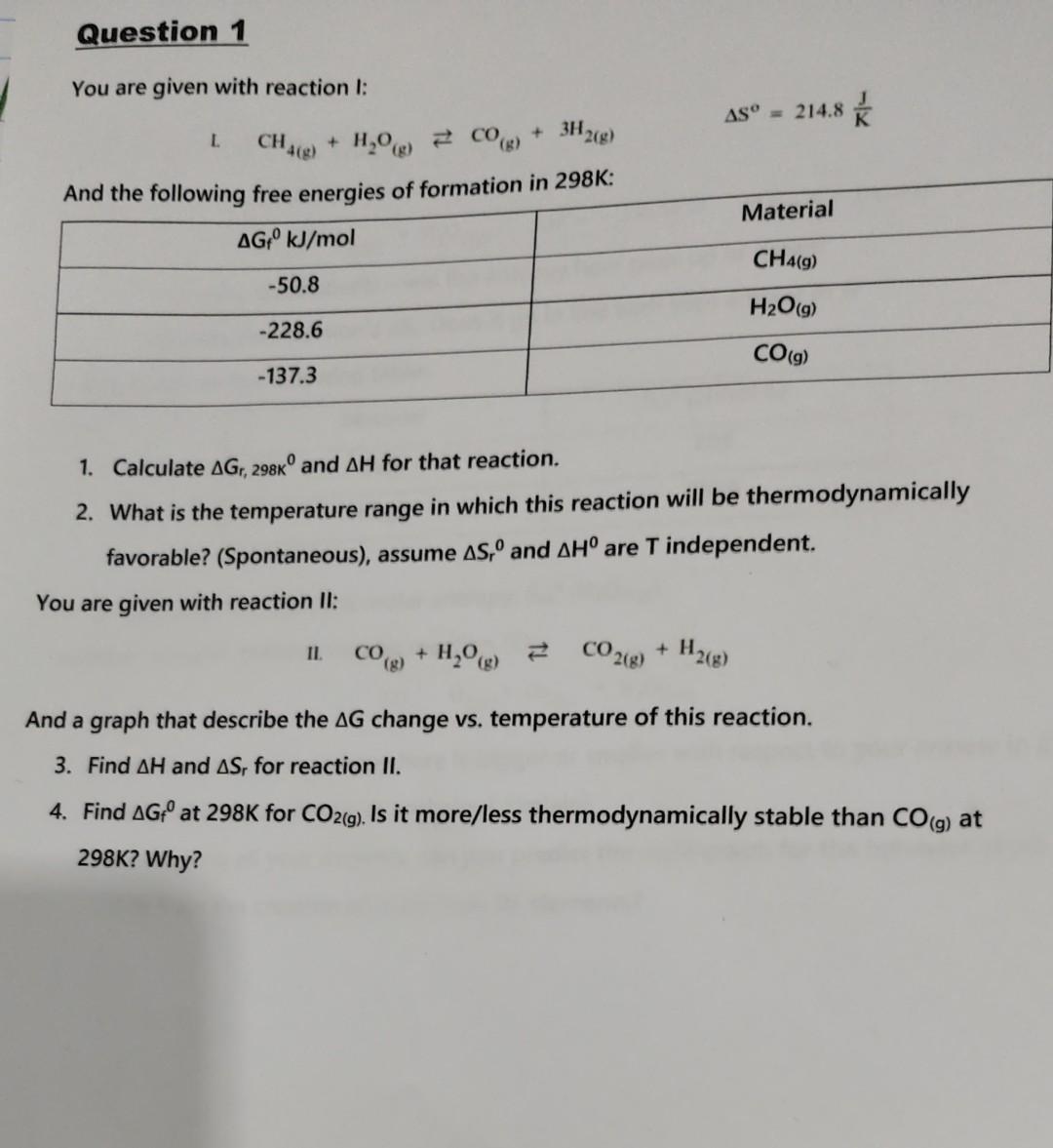
I am attaching 2 Pictures the graph and the Question. Please short explanations of no more than 4-5 sentences Thanks
The graph G change vs. temperature in reaction II: You are given with reaction I: L.CH4(g)+H2O(g)CO(g)+3H2(g)S=214.8KJ And tha fallaniann fran anaraiac of formation in 298K : 1. Calculate Gr,298K and H for that reaction. 2. What is the temperature range in which this reaction will be thermodynamically favorable? (Spontaneous), assume Sr0 and H0 are T independent. You are given with reaction II: II.CO(g)+H2O(g)CO2(g)+H2(g) And a graph that describe the G change vs. temperature of this reaction. 3. Find H and Sr for reaction II. 4. Find Gf0 at 298K for CO2(g). Is it more/less thermodynamically stable than CO(g) at 298K? WhyStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


