Question
I am currently working on calculations for my viscosity of liquids lab report. I have calculated the viscosity of Methanol/Water mixtures of 0%, 20%, 40%,
I am currently working on calculations for my viscosity of liquids lab report.
I have calculated the viscosity of Methanol/Water mixtures of 0%, 20%, 40%, 60%, 80% 100% methanol/water.
Now, I am trying to calculate the Fluidity of the liquids using the following equation:
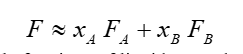
where xA and xB are the mole fractions of liquids A and B, and FA and FB are the fluidities of the pure liquids A and B. The fluidity of the mixture is, therefore, just the sum of the pure liquid fluidities, each weighted by its respective mole fraction.
I have no idea how to calculate the mole fractions for water and methanol just given the percentage of the solution and the individual fluidities.
For example, for my 20% methanol calculation the density of the mixture is 0.971g/mL
Would I find the moles by dividing the volume percentage of the solution by the density? but then how would I calculate the mole fraction?
Thank you in advance.
FxAFA+xBFBStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


