Question
Compound A has a vapour pressure of 350 mm Hg at 95 C whereas compound B has a vapour pressure of 140 mm Hg
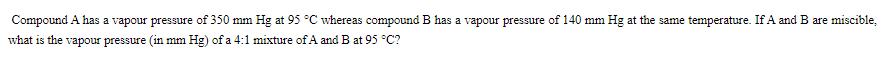
Compound A has a vapour pressure of 350 mm Hg at 95 C whereas compound B has a vapour pressure of 140 mm Hg at the same temperature. If A and B are miscible. what is the vapour pressure (in mm Hg) of a 4:1 mixture of A and B at 95 C?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Modern Advanced Accounting In Canada
Authors: Hilton Murray, Herauf Darrell
7th Edition
1259066487, 978-1259066481
Students also viewed these Business Communication questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



