Question
I AM NOW CONFUSED RIGHT, WHICH ONE IS THE CORRECT ANSWER These are the answers given by experts which one is correct The overall rate
I AM NOW CONFUSED RIGHT, WHICH ONE IS THE CORRECT ANSWER
These are the answers given by experts which one is correct
The overall rate of the reaction correlates to the rate of the slow step, so the third step is referred to as the rate determining step of the reaction. In the above expression, the rate of the reaction is determined solely by the rate constant k (let us consider). The rate of the other steps except third step does not add to the overall rate of the reaction as determined experimentally.
Explanation:
The experimentally determined rate in a multistep reaction correlates to the rate of the slowest step. The rate determining step (or rate limiting step) is the step with the lowest rate number among the other steps of the reaction.
Step 2/3
If the rate constant of step-3 is denoted as k then the rate of the first step in the reaction (and the total reaction) will be,
rate=k[C.S][H.S]3
Explanation:
The rate equation for this reaction is equal to the rate constant of step-3 multiplied by the reactants of that third step.
Step 3/3
The rate of disappearance of CO =(CO)t=k[C.S][H.S]3
Explanation:
rate of disappearance of reactant is always written with negative sign.
Final answer
The rate of disappearance of CO =(CO)t=k[C.S][H.S]3
Was this answer helpful?
second answer
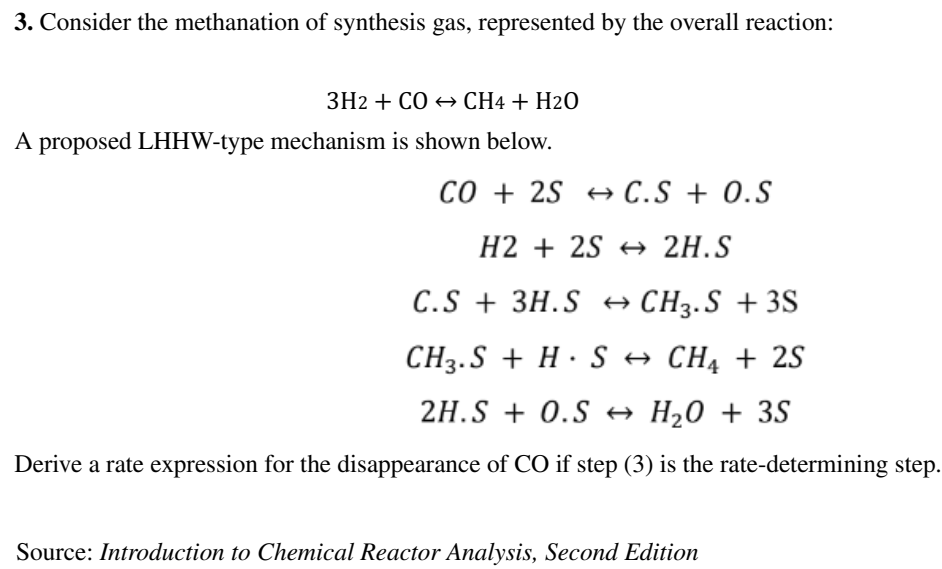
3. Consider the methanation of synthesis gas, represented by the overall reaction: 3H2+COCH4+H2O A proposed LHHW-type mechanism is shown below. CO+2SC.S+O.SH2+2S2HSC.S+3H.SCH3.S+3SCH3.S+HSCH4+2S2H.S+O.SH2O+3S Derive a rate expression for the disappearance of CO if step (3) is the rate-determining step. Source: Introduction to Chemical Reactor Analysis, Second EditionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


