Answered step by step
Verified Expert Solution
Question
1 Approved Answer
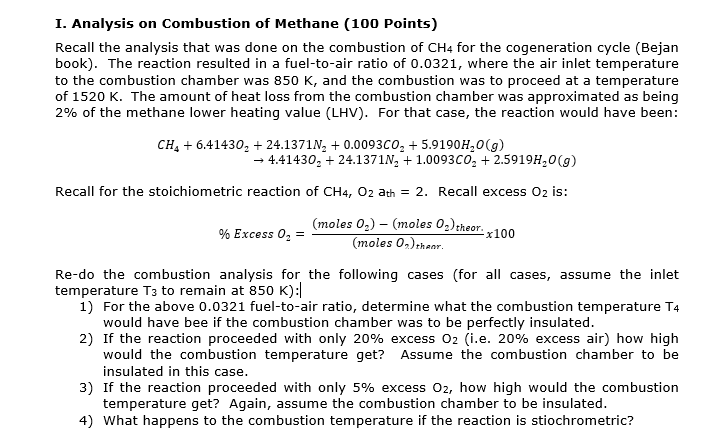
I. Analysis on Combustion of Methane ( 1 0 0 Points ) Recall the analysis that was done on the combustion of C H 4
I. Analysis on Combustion of Methane Points
Recall the analysis that was done on the combustion of for the cogeneration cycle Bejan
book The reaction resulted in a fueltoair ratio of where the air inlet temperature
to the combustion chamber was and the combustion was to proceed at a temperature
of The amount of heat loss from the combustion chamber was approximated as being
of the methane lower heating value LHV For that case, the reaction would have been:
Recall for the stoichiometric reaction of ath Recall excess is:
Excess
Redo the combustion analysis for the following cases for all cases, assume the inlet
temperature to remain at :
For the above fueltoair ratio, determine what the combustion temperature
would have bee if the combustion chamber was to be perfectly insulated.
If the reaction proceeded with only excess ie excess air how high
would the combustion temperature get? Assume the combustion chamber to be
insulated in this case.
If the reaction proceeded with only excess how high would the combustion
temperature get? Again, assume the combustion chamber to be insulated.
What happens to the combustion temperature if the reaction is stiochrometric?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


