Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(i) Calculate the double bond equivalents (DBE) for each molecule. (ii) Assign the 'H NMR spectra completely, rationalise the number of peaks, relative integral,
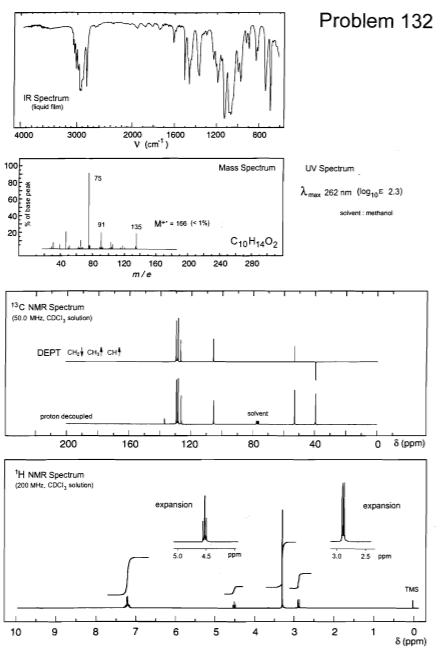
(i) Calculate the double bond equivalents (DBE) for each molecule. (ii) Assign the 'H NMR spectra completely, rationalise the number of peaks, relative integral, their multiplicity or splitting pattern where appropriate. (iii) Rationalise the 13C NMR spectra. (iv) Rationalise all numbered peaks in the mass spectra and IR spectra. (v) Provide a brief logical explanation for how you arrived at unambiguous structures for compounds A and B. Problem 132 IR Spectrum (iquid fim) 2000 1600 V (cm") 4000 3000 1200 800 100 Mass Spectrum UV Spectrum 75 80 Ame 262 nm (log,E 23) scivent methanal 91 M- 100 < 1% 135 40 80 120 160 200 240 280 m/e 13C NMR Spectrum (50.0 MHz, CDCI, souson) DEPT CH Cnt cHt solvent proton decouplied 200 160 120 80 40 8 (ppm) TH NMR Spectrum (200 Mitz. CDCI, solution expansion expansion 5.0 45 ppm 10 25 ppm TMS 10 6 3 2 8 (ppm)
Step by Step Solution
★★★★★
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


