Answered step by step
Verified Expert Solution
Question
1 Approved Answer
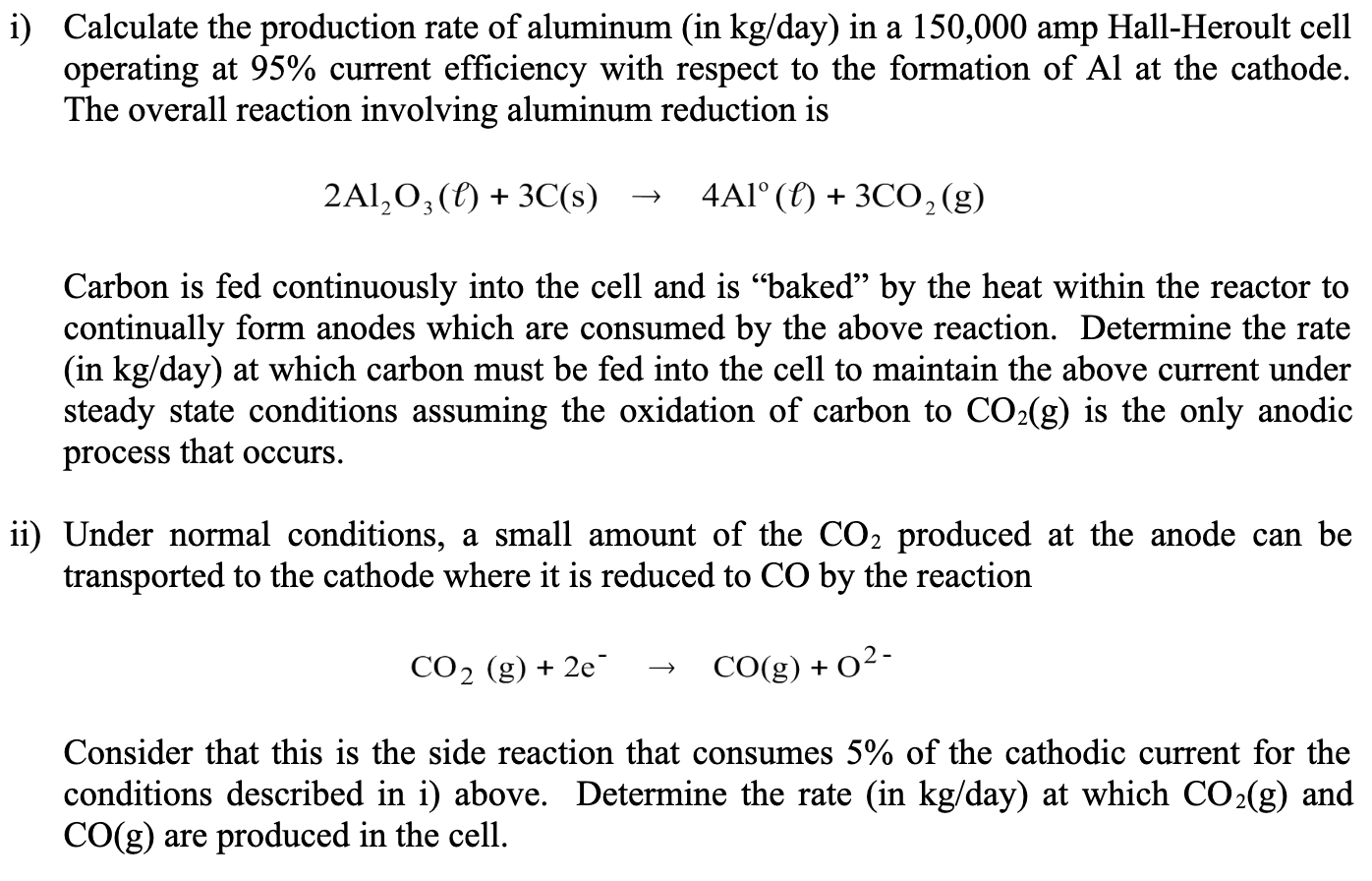
i ) Calculate the production rate of aluminum ( in k g / day ) in a 1 5 0 , 0 0 0 amp
i Calculate the production rate of aluminum in day in a amp HallHeroult cell
operating at current efficiency with respect to the formation of at the cathode.
The overall reaction involving aluminum reduction is
Carbon is fed continuously into the cell and is "baked" by the heat within the reactor to
continually form anodes which are consumed by the above reaction. Determine the rate
in day at which carbon must be fed into the cell to maintain the above current under
steady state conditions assuming the oxidation of carbon to is the only anodic
process that occurs.
ii Under normal conditions, a small amount of the produced at the anode can be
transported to the cathode where it is reduced to by the reaction
Consider that this is the side reaction that consumes of the cathodic current for the
conditions described in i above. Determine the rate in day at which and
are produced in the cell.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


