Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i cant seem to find the correct rate of cooling, can someone please help? 29.0 C Forml: Cp kJ/mol.) or (kJ/(mol)] = a +bT +
i cant seem to find the correct rate of cooling, can someone please help? 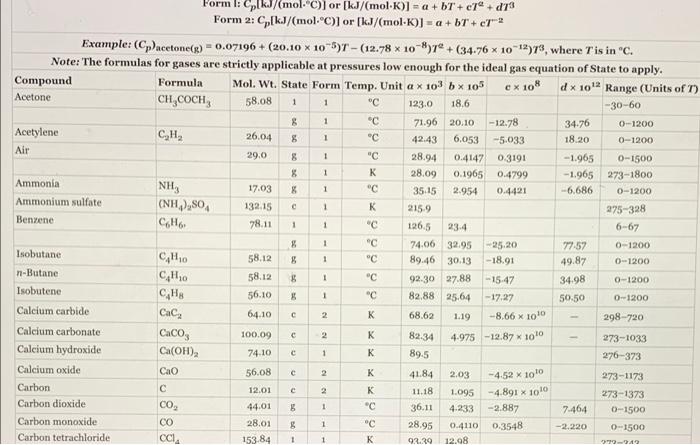

29.0 "C Forml: Cp kJ/mol.") or (kJ/(mol)] = a +bT + c7" +d78 Form 2: C[kJ/(mol-C)) or (kJ/(mol-K)] = a +bT+ ch2 Example: (Cpacetone(s) - 0.07196 + (20.10 x 10-5) - (12.78 x 10 ) +(34.76 x 10-12)7%, where T'is in "C. Note: 'The formulas for gases are strictly applicable at pressures low enough for the ideal gas equation of State to apply. Compound Formula Mol. Wt. State Form Temp. Unit a x 103 bx 105 e x 108 x 10 Range (Units of T) Acetone CH,COCH 58.08 1 1 "C 123.0 18.6 -30-60 8 1 "C 71.96 20.10 -12.78 34.76 0-1200 Acetylene CH, 26.04 8 1 "C 42.43 6,053 -5.033 18.20 0-1200 Air 8 1 "C 28.94 0.4147 0.3191 - 1.965 0-1500 % 1 28.09 0.1965 0.4799 -1.965 273-1800 Ammonia NH, 17.03 X 1 35.15 2.954 0.4421 -6.686 0-1200 Ammonium sulfate (NH),SO 132.15 1 215.9 275-328 Benzene CH 78.11 1 "C 126.5 6-67 8 1 74.00 32.95 25,20 77.57 0-1200 Isobutane 58.12 8 1 89.46 30.13 -18.91 49.87 0-1200 n-Butane CH10 58.12 8 1 "C 92.30 27.88 -15-47 34.98 0-1200 Isobutene CHA 56.10 "C 82.88 25.64 - 17.27 50.50 0-1200 Calcium carbide Cac, 64,10 c 2 K 68.62 1.19 -8.66 x 1010 298-720 Calcium carbonate , 100.09 C K 82.34 4.975 -12.87 x 1010 273-1033 Calcium hydroxide Ca(OH)2 74.10 C 1 K 89.5 276-373 Calcium oxide Cao 56.08 2 K 41.84 2.03 273-1173 Carbon 12.01 2 K 11.18 1.095 -4.891 x 1010 273-1373 Carbon dioxide CO 44.01 1 " 36.11 4.233 -2.887 7.464 0-1500 Carbon monoxide CO 28.01 & 1 "C 28.95 0.4110 0.3548 -2.220 0-3500 Carbon tetrachloride 153 84 1 K 92.39 12.08 3 - 4 * * * 1 $ 1 c c -4.52 x 1010 c Cla - 1994 A stream of carbon monoxide flowing at 350.0 kg/min is cooled from 425.0C to 25.0C at a low pressure. Physical Property Tables Cooling Required Calculate the required magnitude of the rate of cooling (kW), using the enthalpy function of APEx. Check the calculation using the heat capacity formula in Table 8.2. 11 kW 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


