Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I can't seem to understand how they got this answer. Part A How many moles of N,Os will remain after 70 min? Express the amount
I can't seem to understand how they got this answer. 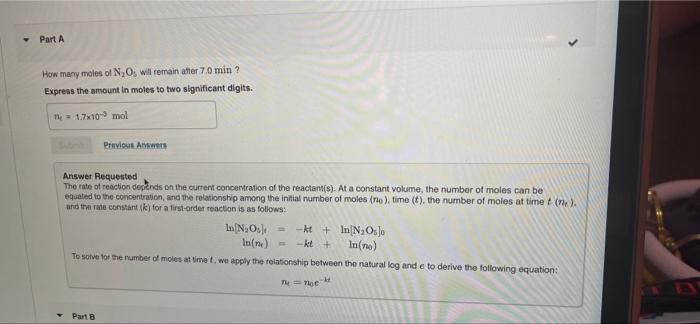
Part A How many moles of N,Os will remain after 70 min? Express the amount in moles to two significant digits. ni 17x10 mol Previot Awes Answer Requested The rate of reaction depends on the current concentration of the reactant(s). At a constant volume, the number of moles can be equaled to the concentration, and the relationship among the initial number of moles (no), time (t), the number of moles at time (ne). and the rate constant (k) for a first-order reaction is as follows: In N,Os) -kt + In N Oslo In(ne) - kt + In(no) To solve for the number of moles at time t, we apply the relationship between the natural log and eto derive the following equation: = Moe Part 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


