I completed the experiment. I just need help with the "Determinations." Thanks
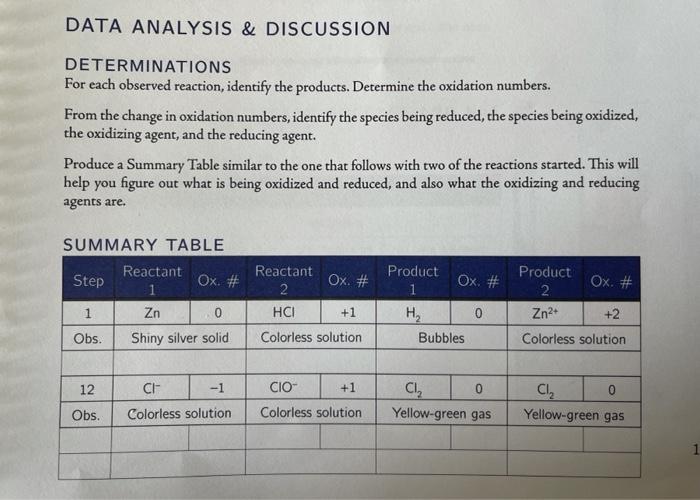






DATA ANALYSIS & DISCUSSION DETERMINATIONS For each observed reaction, identify the products. Determine the oxidation numbers. From the change in oxidation numbers, identify the species being reduced, the species being oxidized, the oxidizing agent, and the reducing agent. Produce a Summary Table similar to the one that follows with two of the reactions started. This will help you figure out what is being oxidized and reduced, and also what the oxidizing and reducing agents are. SUMMARY TABLE Reactant Product Product Step Ox. * Ox. # Ox. # Ox. # Reactant 2 HCI 1 Zn 0 +1 0 Hy Bubbles Zn2+ +2 Colorless solution Obs. Shiny silver solid Colorless solution 12 CH- -1 CIO +1 CI 0 Yellow-green gas Cl 0 Yellow-green gas Obs. Colorless solution Colorless solution CHEMICAL ALERT: Formula Hazard Torcic and corrosive HCI Chemical Name Hydrochloric acid Zinc (mossy) Magnesium (turnings) Zn Low hazard Mg Benedict's reagent Flammable solid Toxic, irritant, and aquatic environmental hazard Low hazard Glucose Potassium oxalate CuSO:/Na,Co,/Na,H,O, SHO KO, HASO, Sulfuric acid Potassium permanganate KMnO Sodium hydroxide NaOH Bleach (sodium hypochlorite) NaCIO Irritant, harmful if swallowed Toocic and corrosive Corrosive and oxidant, discolors skin and stains clothing. agauatic environmental hazard Toxic and corrosive Corrosive. aquatic environmental hazard Toxic and irritant Irritant and aquatic environmental hazard Toxic by ingestion and aquatic environmental hazard Low hazard Dichloromethane CHC Sodium iodide Nal Nakso, Sodium bisulfite or sodium hydrogensulfite Sodium bromide NaBr PART I: REACTIONS OF METALS WITH HCI 1. Add a small piece of mossy Zn (the solid Zn) to a small test tube. Add approximately 2 ml of 10% HCl to the test tube. Observe the reaction and the speed of the reaction. Look for changes in color at the surface of the solid. formation of a gas, and reduction in the mass of the solid. 2. Add a few pieces of the Mg turning to a second small test tube. Add approximately 2 mL of 10% HCl to the test tube. Observe the reaction and the speed of the reaction. You may have to wait up to 5 minutes. Verify the product(s) formed. H.(g) is a colorless gas. Zn"(aq) and Mg"(aq) are colorless solu- tions. For the reaction in step 1, what is the oxidation number of Zn(s)? What is the oxidation number of Zn in Zn?"(aq)? What is the oxidation number of H in HCl(aq)? What is the oxidation number of H in H (g)? For the reaction in step 2, what is the oxidation number of Mg(s): What is the oxidation number of Mg in Mg(aq)? 3. Any solid pieces of metal remaining should be placed in the solid waste beaker in the hood. Discard all of the solution down the drain with lots of running water. VARIOUS REDUCTION-OXIDATION REACTIONS PART II: REACTION OF Cu2+ WITH GLUCOSE The reaction of Cu?with glucose is the basis of a test formerly used by diabetics to test blood sugars. Benedict's reagent (Cult in a basic solution) reacts as shown in the reaction below. CH,0(aq) + 2 Cu?*(aq) + 6H20(e) Cu,C(s) +CH,,0_(aq) + 4 H,0"(aq) 4. Prepare a hot water bath by filling a 250 mL beaker approximately half full with water and plac- ing it on a hot plate. Bring to a boil. 5. Add a small amount of glucose (enough to cover the tip of the spatula) to a small test tube. Add 2 mL of deionized water to the test tube and stir until the glucose dissolves. 6. Add 2 mL of Benedict's reagent to the glucose solution. Observe the reaction. Heat the rest tube in the hot water for 5 minutes. Observe the reaction. Verify the product(s) formed. Copper(II)oxide (CuO) is a black solid. Copper(1)oxide (Cao) is a red solid. Cu?"(aq) is a blue solution.CH,, (aq) and C,H,O,(aq) are colorless solutions. What is the oxidation number of Cu in Cu?*(aq)? What is the oxidation number of Cu in the product? Is the Cu oxidized or reduced? Is the C being oxidized or reduced 7. Pour the contents of the test tube in the liquid waste container in the hood. PART III: REACTIONS OF Mno. 8. Add 1 mL of 0.1 MK.C_0, to a small test tube. Then add 10 drops of 6 MH.SO, to the test tube. Stir to mix. 9. Add 1-2 drops of 0.1 M KMnO, to the solution. Stir to mix. If there is no change, then heat in the hot water bath for 1 minute. Observe the reaction. 10. Add 3 mL of 0.1 M NaHSO, to a second small test tube. Then add 1 mL of 10 M NaOH to 1 the test tube. Stir to mix. 11. Add 1-2 drops of 0.1 KMnO, to the solution. Stir to mix. If there is no change then heat in the hot water bath for 1 minute. Observe the reaction. Pour reaction mixture in liquid waste container in hood. Verify the product(s) formed. MnO, (aq) is a dark purple solution. Mn(aq) is a very pale pink to nearly colorless solution. Manganese(IV) oxide (MnO) is a black solid. CO (g) is colorless gas. HSO, (aq), so (aq), and C,0,-(aq) are colorless solutions For the reaction in steps 8 and 9, what is the oxidation number of Cin Co. (aq)? What is the oxidation number of Cin the product? What is the oxidation number of Mn in MnO,-(aq)? What is the oxidation number of Mn in the product? For the reaction in steps 10 and 11, what is the oxidation number of Sin HSO,- (aq)? What is the oxidation of Sin the product? What is the oxidation number of Mn in MnO,- (aq)? What is the oxidation number of Mn in the product? EXPERIMENT 20 PART IV: REACTIONS OF CIO + HCI (FORMS CI) For this set of reactions, wear gloves and do not inhale the fumes from the test tubes! Do not use Beral pipet to measure or dispense the CHCI) Use the dropper provided in the reagent bottle 12. Add 5 drops of water to a clean, small test tube. Add 20 drops of dichloromethane (CH,CI,) to the test tube Add 10 drops of 6 M HCl to the test tube. Next, add 1 drop of bleach solution (5% NACIO) to the test tube. Shake the test tube from side to side for about 1 minute. If the solution isn't yellow, then add 4-5 more additional drops of bleach and shake again. Record all observations. Set the test tube aside. NE Figure 20-1. Technique for Mixing Solution by Shaking Test Tube 13. Add 5 drops of 1.0 M NaBr, 20 drops of dichloromethane (CH.CI), and 10 drops of 6 M HCI to a clean, small test tube. Record your observations 14. Add 1 drop of bleach solution (5% NaCIO) to the test tube. Shake the test tube from side to side for about 1 minute. Add 4 more drops of bleach solution. Record all observations. Place test tube aside. 15. Add 5 drops of 1.0 M Nal, 20 drops of dichloromethane (CH,CI,), and 10 drops of 6 M HCI to a dean, small test tube. Record your observations. 16. Add 1 drop of bleach solution (5% NaCIO) to the test tube. Shake the test tube from side to side for about 1 minute. Add 4 more drops of bleach solution. Record all observations 100 a 17. Add an additional 10 drops of bleach solution to the test tube. Shake the test tube for 1 minute. Hold the test tube at a slight angle when shaking. You need to make sure all the solution is mixed together well. Then let the solution sit in the test tube rack for an additional minute. Record all observations. Verify the product(s) formed. Cl is colorless solution; Cl, is yellow-green gas that dissolves in water and in CH,CI. Bromine, Bry, is an orange liquid that dissolves in CH,CI; Br (aq) is a colorless solution. 12(aq) in the presence of excess I forms a red-brown solution. Iz(s) dissolves in CH,Cl, to form a purple solution. 12(s) is dark purple (almost black). In step 12, there is a reaction of CIO with Cl producing Cl2(g). This is a conproportionality reac- tion, where both of the half reactions form the same product. The overall reaction is given below. 2 H(aq) + CI+(aq) + CIO" (aq) C12(g) + H2O(e) In steps 13 and 14, you once again form Cl2(g) first. Then the Cl, reacted with the NaBr. What is the oxidation number of Clin Cl? What is the oxidation number of Cl in the product? What is the oxidation number of Br in NaBr? What is the oxidation number of Br in the product? In steps 15 and 16, you once again form C2(g) first. Then the Cl, reacted with the Nal. What is the oxidation number of Cl in Cl? What is the oxidation number of Cl in the product? What is the oxidation number of I in Nal? What is the oxidation number of I in the product? a In step 17, the iodine product from step 16 further reacts with additional Cl, in another reaction to produce 10,- (aq), a colorless solution. What is the oxidation number of I in IO,-? a Cutz + Ze Cucg). Champws 2n - 2n + Lent 2e PRATIS After few min, it turned black. The solid peice of mossy Zndid not change color on the surface. Bubbling occurred as the solid appeared to be dissoluing, at a slow rate. -The solid peices of mg did not change color on the surface. Bubbing occured immediately as the solid appeared to be dissolving at a faster rate. Hz confirmed, 2n+2 indicated, Mgar confirmed present. The oxidation number of 2n(s) and Znat is 2. The oxidation number of Hin Holland Hrig) is o.. The oxidation number or Macs) and my is Ba. PARIZ. - After 2ml of Benedict's Reagent was added to the slucose solution, no apparent reaction occured Colors remained the same, no bubbling, etc. After heating test tube, the solution turned green then brown then Ororge then red. -Cuo and Coat was prescut After a few more more of heating, the solution turned black indicating the Presence of cuo. -Cuin Cult is a and the oxidation number of Cu in the product is o. Co is being reduced; C is being oxidized. 44) so you, is 3 Continued... Pers! After KMnO4 is added, the solution turned dark pink then clear. 3 ml of Nattso, I m of NaOH, and a few more drops of KMnO4 are added. The solution. turns red at top and clear at botton then brownish yellow at top and clear at the bottom. -M-ot is indicated. 4503 can), and C0 ans are confirned. -c in cox is 3 m in nor is 3.c in product is 3. Main product is 3 oxidation in H Sox is 3.sin product is 3. The oxidation number of Mr in M-04 and Mn in the product is 3. Pher 4: After 5 drops of water, 20 drops or CHCl2, 10 drops of Hol, and b drops of bleach, the solution turned yellow. Het made the solution have Some sert of bubble in the middle. -Test tube 2: HCI made the solution have some sort of bubble in the middle. I drop of bleach caused the bubble to turn dark orange and the rest of the solution to be light orarse. 4 drops of bleach couses a red solidl liquid on the bottom and a yellow solution on top. - Test tube 30 NAI, CHCl2, and tich is a liquid with bubbles. I drop of bleach turned it dark red, 4 more drops created a dark black solid at the bottom, and a yellow solution in the middle with purple solution on top and botton. 10 more drops of bleach formed a block solid at the bottom with a cloudy solution on top. -Br , Iz, and clz is confirmed. oxidation numbers BI in NaBr 0 Br in producto CI = 0 producto NAT-O product O TO3 = 2 Product=2 a JHH - OTHH













