I done pages 1-4 but not 5-6 please help me that one. Don't do pages 1-4 like last time, because wasted 3 questions already.

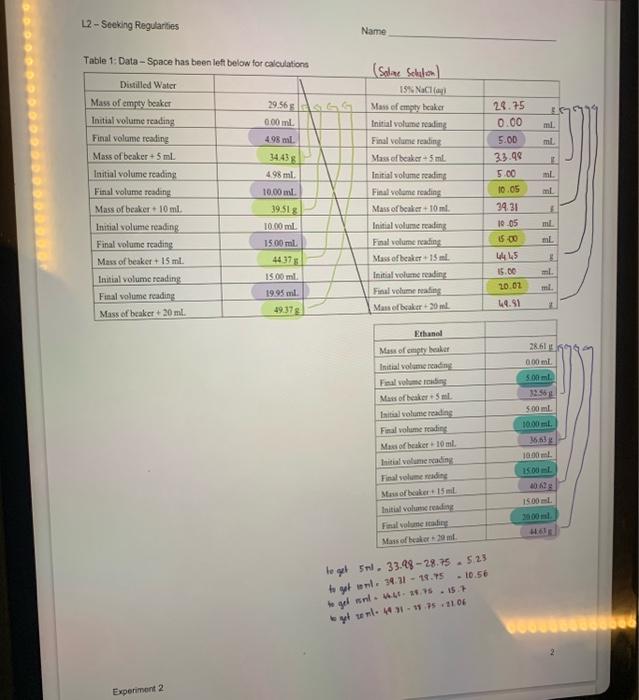
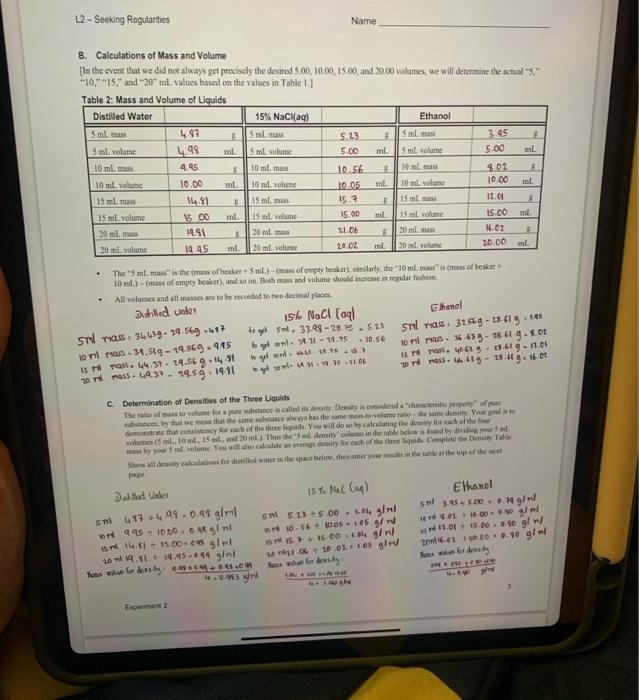
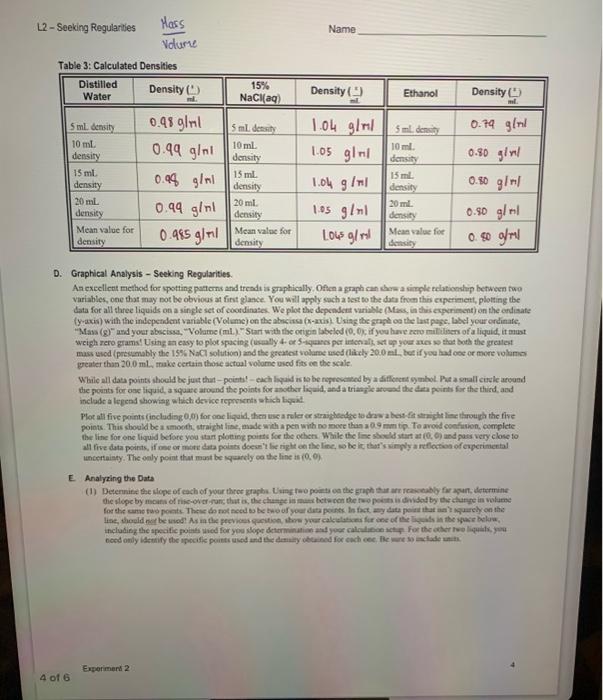
Introduction A big part of scientific investigation involves looking for patiems in the behavior of substances. Offen it mesns measuring bow ene property of a sabstance changes in response to a charge in soncthing else. This cia be accomplished by plottiag the changss in techavior. In this experiment we will measure the change in mass that accompanies changes in the volume of material fis three different liquids, recording the change in mass that accompanies fixed increases in the volume of liquid. Uaits of neasuremeat are always important. For this exercise, mass will te measured in grams (g), and volame wial be ia millitisis (mL). The uncertainty in the mass on our electronce balences is =0.01g, so all masees are to be reported to two decimal places. You will be intruduced to a pioce of laboratory apponatus that you may never have sees befoec the bere. Typically, burcts are calitratod in tenths of a miltiliter. You are epperted to cuimate betwon limes, at lesst to a precsion of +0.05mL. This means that all wolames will also be recorded to two decimal places. Your instuctor will demenstrate the proper use of the bure. Tbe three liquids to be used are distilled water, ethanol, and a 15% saline solution, sodram chlanide, NaCl, disolued in water. The directions below are the same for all three liquids The orler in which you do the liqaids does eot maner, so do the one that is most readily available at the time; you will do all three liquids during the lakeratory period. Procedure An empty, cleas 50-mL. beaker will be used as the centainer. It will be weighed alone, then with increaing amounts of liquid. Use the buret to carefully add exactly 5.00mL of tiquid to the beaker, then weigh it again. In he data uble record both the initial and final readings on the buret scale; the difference between the final and witial readings is the volume added. You dos' have to ger 5.00 mL exactiy, so long as the actnal initial and final maings are recondnd. An adcitional 5.00mL. of liquid is addod to the liquid already pesent. We reoond the initlal and final turet readings, then weight. beaker and coatents chee again. The process is repeated twice more, winding up with a fotal of 20.00ml. of liquid to the beaker. Repear the procedure with each of the other two liquids, staning with a clean beaker caih time. Added note. While the experiment itself is very quick and esily performed, the calcolarions and analysit of results will cake some time, care, asd careful thought. In completing the tables on the next page, bear in mind that it is the sotal mass and toeal volume that are te be reecelod. This mans that the values should iacteases steadily. A 15% solution means that 100 . grams of eolution centains 15 gram of solute (ant 35 grams of eotienin) Table 1: Data - Space has been left below for calculationa B. Calculations of Mass and Volume (In the event that we cid not always get precisely the desirod 5.00,10,00,15.00, and 20.00 volumes, ue will delermine the actial "5," "10," "15," and "20" mL, values based on the values in Table.1.] 10mL) - (rass of emply beaker), and io on. Pofl mass aed volume shodd ecrese ia nggalar fuction. - All volutassand all mase are to be recoeled to two decimal plosen. Duthlicd valer c. Determination of Densities of the Three Lquids 3elnid bines I 5sthl5.23+5.00+1.04glnl3nl35+500=0.79g/rd Tahle 3: Calculated Nancitioc: D. Graphical Analysis - Seeking Regularities. An exeelleot methed for spotting patoms and treadt is graphically. Oficn a graph can shere a simple relationslep between two variables, one that may pot be obvious at firit glance. You will apply sach a test to the data from this ceperiment, plotting the Lata for all three liquids on a single set of coordinases. We plot the depcndent varible (Maxs, in this experiment) on the endisate (y-axis) with the indeperadent variable (Vedeme) on the abscisa (x-2xis). Usiag the grapk oe the las page, labet your erdinate, weigh rero grams! Using aa easy to plot spacing (usmally 4- er S-aquates per irten als, st up your anes wo that both the greateot mass sued (presimably the 15%NaCl solution) and the greatest volime ased (hitely 20.0al but if you had coe or more volims greater than 20.0ml, thake certaia thase actoal volame used fits oe the seale. the points for ose liquid, a squate aroend the points for asother lig aid, anda triapite arvead the dea points for the thicd, asd inclade a legend showiag welich device reprosents a hich hewit. Plot atl five points (including 0.05 for one liquid, then use a reler er straigheedye to dras a bet fi straicht line through the five points. This showld be a smonth, atraight lise, made which a pen with no moee than a 99 man tip. To anoid confinion, complete the line for one liquid before you itar photing points foe the ohen. While the line sheld start at (0,0) and pass very close to all fire data points, if one of mete deta poisisi dsese't he right en the line, so be ic that's vingly a reflection of experimertal uncertaiaty. The only point that mant be squardy ea the line is (0.0). E. Analyzing the Data the diepe by moara of tikeover : Name: (2) The slepes you calculaed in Qustion ( (1) are the dentitics of the frer hiywidc. Eipiais why this is so fleint what are the units of your slopes?? (3) Fhanol is a highty volatile liquod, that is, it craponacs readty. low, of unaffectod? Defecod your choice. (b) How might if affect the valoes you obtain fer the 5., 10-, 15., and 20-1. velumes? Be specific- will they be too high, too low, or unaffected? Deficnd your choice. (c) How might cthanol's velatility affect the avenge valueyou oberisod for its denaity? Be secific: witl it be toe highi, too low, or inaffected? Defced your choke. "For the groph oo the next page, it will probably wod to hwe 5 of the malket drision equal 1.0 gee the vertical (y) auia, and 5 of the smallest divisions equal to /mL en de hocimontal (s) atis hot check to make certain it mill fi before label ing your greph. Whatcver scale you use, expect to have to estinate where you plice the points. L2- Socking Regularities