Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i dont know how to find the percent error can someone explain I need to find the percent error and water density and im kinda
i dont know how to find the percent error can someone explain 


I need to find the percent error and water density 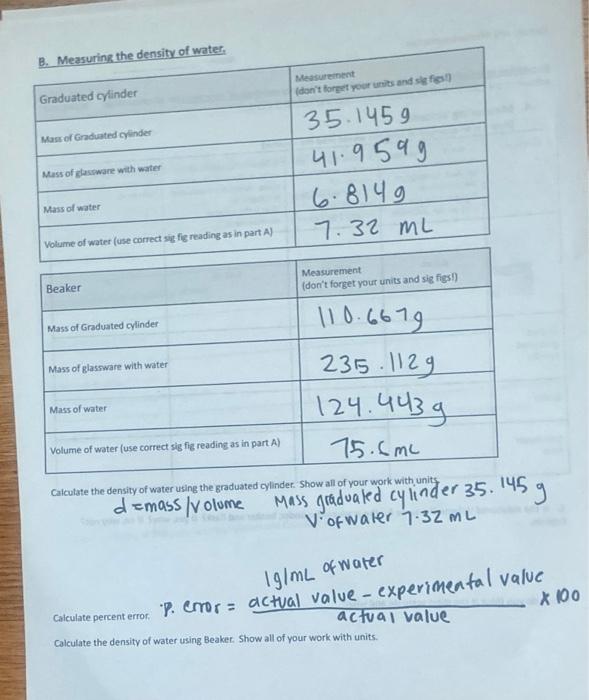
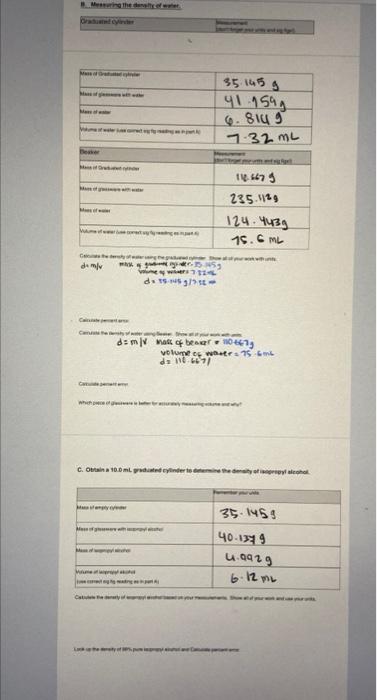
0. Detertnining the Deneity of Wainer 1. Take yosr graduated oyfinder been pant A. Wigh the gradcated cyindar and racond the mass to the oortect fumker: of signiticant figures. 2. Fil the of asswarn roughly 231 il with deionized water asd roord the mass. The anainf of wakter is nor inporkanf do not try to fiv exacfiy to at kinef 3. Fead the voluine of the water to the comect number of sionifeset flgures. Use volume and mass to-caiculate the density ef walet. -4. Find the percerta ertot thom the acduid densht of walar (1. 00gmi.) for your measurement. PercentEirtor=100% actustvilueactuatvaueyourexpermentatvaluel| 5. Take the bebker that you ised in pert A. dryt, whights and recond the moss to the corfect number at significari: figures 6. Fil the glasseate roughly 25 tuli with deionizad aater and seased the mass. The amount of watar is not inporlanfdo not dey oxacty to a ine! 7. Aead the volume of the water bo the conect number of tignificant figures. Use volume and mass to caloulate the density of walet. 4. Find the percent error from the acted densily of war (t 89gm L. igr your meas urement. Q. Which peece of giassware is tetler lor menturing voi atte and aty? C. Determining the density of istpropylalcohol 1. Obtain wash and carefully dfy a 10.0mL graduabed tyirder and woigh it. Peoond the mass so the correct numbar of eignificant ficures. 2. Fill the cyinder to at least 2/3 ful with isppropyl alcohel and teephh. Aecera the mass. Jne ambunf of iscprcpt aicahol is nof inportent - do nof iny to fw exacty to a linel 3. Read the volume of the isopropyl alsohol to the corsect iue oer of uigniceant foures. Use volume and mass 10 : calculide the density of isopropyl alcohol. This will be your erperimantal value. Los in the moty B. Measuring the density of water. \begin{tabular}{|l|l|} \hline Beaker & Measurement(dortforgetyourunitsandsigfigsi) \\ \hline Mass of Graduated cylinder & 110.63 \\ \hline Mass of glassware with water & 123 \\ \hline Mass of water & 23 \\ \hline Volume of water (use correct sig fig reading as in part A) & 15.13 \\ \hline \end{tabular} Calculate the density of water using the graduated cylinder. Show all of your work with units demass/volome Mass gradualed cylinder 35.145g Vofwater 7.32mL 1g1mL of water Calculate percenterror. Pror =actvalvaluedctualvalue-cxperimeatalvalue Calculate the density of water using Beaker. Show all of your work with units and im kinda confused on how to

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


