Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I don't need an explanation for any of the questions just the correct answers, thank you 1. [1A] A musician mentioned that part of her


I don't need an explanation for any of the questions just the correct answers, thank you
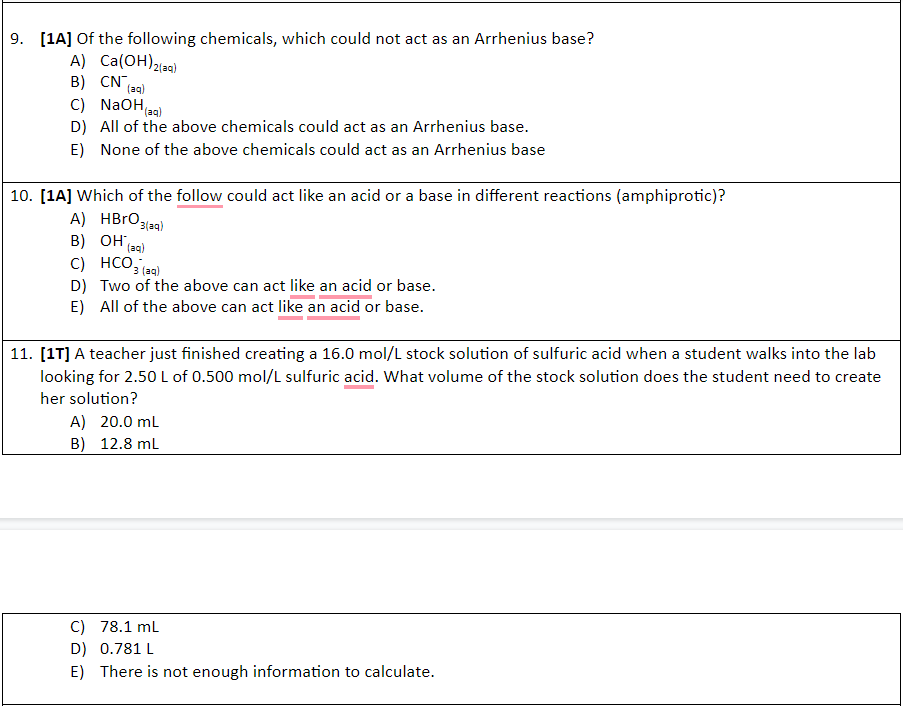
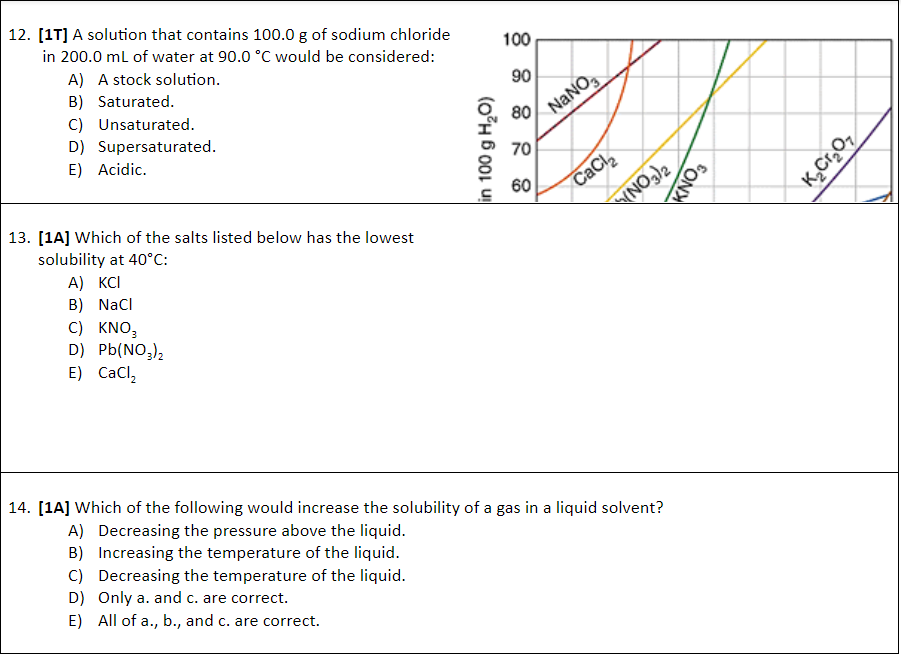
1. [1A] A musician mentioned that part of her superior sound quality was due to her brass tuba, which was made of 70% copper and 30% zinc. Which of the following statement is true based on this information? A) The solute in this brass tuba is copper. B) The solute in this brass tuba is zinc. C) The solvent in this brass tuba is zinc. D) The solution in this tuba is copper. E) There is no such thing as a solution made of metals. 2. [17] Which of the following would likely be the least soluble in water? A) Aluminum hydroxide. B) Ammonia. C) Sodium hydroxide. D) Sodium chloride. E) All of the above would be equally soluble in water. 3. [17] Which of the following chemicals would remain mostly intact after being mixed with water? A) Sodium chloride. B) Ammonia. C) Lithium nitrate. D) Potassium hydroxide. E) All of the above chemicals would dissociate in water to form ions. 4. [17] Scientists determine that in a cool, fast moving river, 112.0g of oxygen gas are dissolved in 10.0L of cold water. Based on this information, the amount concentration is approximately: A) 11.2 mol/L B) 70.0% C) 3.50 mol/L D) 0.350 mol/L E) 0.700 mol/L 5. 1T] If the water in the previous question was heated, how would the concentration of oxygen gas be affected? A) More oxygen gas would dissolve, increasing the concentration. B) More oxygen gas would dissolve, decreasing the concentration. C) Less oxygen gas would dissolve, increasing the concentration. D) Less oxygen gas would dissolve, decreasing the concentration. E) Changing the temperature would have no effect on concentration. 6. [1T] You have 100.0 mL of a 16.0% (m/v) methanol solution, CH,OH. What is the amount concentration of your solution? A) 4.99 mol/L B) 1.60 mol/L C) 0.499 mol/L D) 0.160 mol/L E) None of the above is the concentration of your solution. 7. [14] The percent concentration (v/v) of a solution that contains 5.00 mL of acetic acid in 20.0 L of solution is: A) 2.50 x 104% B) 0.250 mol/L C) 0.0250 % D) 25.0 % E) 4.00% 8. [1A] What mass of mercury, in grams, is there in a 160.0 kg dolphin, if the concentration is 42.Oppb? A) 6.72 g B) 6.27 x 10g C) 2.625 x 105 g D) 2.625 x 10 g E) None of the above is the correct mass. 9. [1A] Of the following chemicals, which could not act as an Arrhenius base? A) Ca(OH)2laa) B) CN (aq) C) NaOH, D) All of the above chemicals could act as an Arrhenius base. E) None of the above chemicals could act as an Arrhenius base (aq) 10. [1A] Which of the follow could act like an acid or a base in different reactions (amphiprotic)? A) HBrO 3/29) B) OH (ag) C) HCO3 (24) D) Two of the above can act like an acid or base. E) All of the above can act like an acid or base. 11. [17] A teacher just finished creating a 16.0 mol/L stock solution of sulfuric acid when a student walks into the lab looking for 2.50 L of 0.500 mol/L sulfuric acid. What volume of the stock solution does the student need to create her solution? A) 20.0 mL B) 12.8 ml C) 78.1 mL D) 0.781 L E) There is not enough information to calculate. 100 90 12. [17] A solution that contains 100.0 g of sodium chloride in 200.0 mL of water at 90.0 C would be considered: A) A stock solution. B) Saturated. C) Unsaturated. D) Supersaturated. E) Acidic. 80 NaNO, in 100 g H,0) 70 400 60 CaCl KNO3 VNO3)2 13. [14] Which of the salts listed below has the lowest solubility at 40C: A) KCI B) Naci C) KNO, D) Pb(NO3)2 E) CaCl2 14. [14] Which of the following would increase the solubility of a gas in a liquid solvent? A) Decreasing the pressure above the liquid. B) Increasing the temperature of the liquid. C) Decreasing the temperature of the liquid. D) Only a. and c. are correct. E) All of a., b., and c. are correctStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


