Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I figured out The molar Flow rates for D and B already but I'm having trouble with the Qc and Qb values. Something must be
I figured out The molar Flow rates for D and B already but I'm having trouble with the Qc and Qb values. Something must be off with the values of the Heat Capacities that I'm using. Could you give me a pointer?
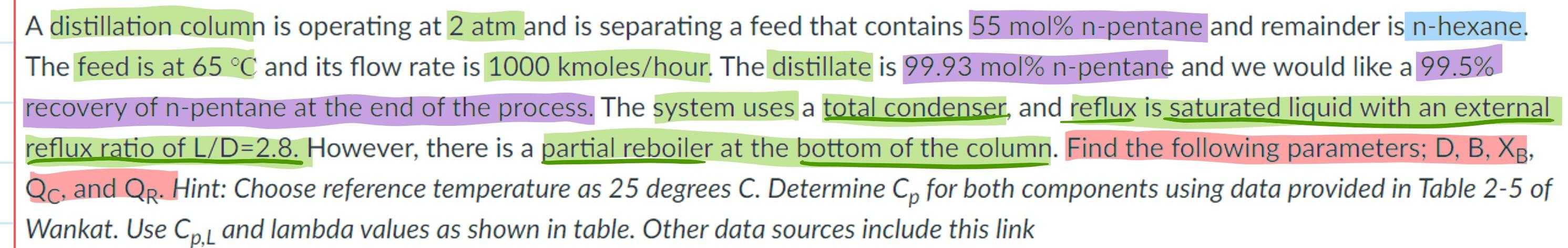
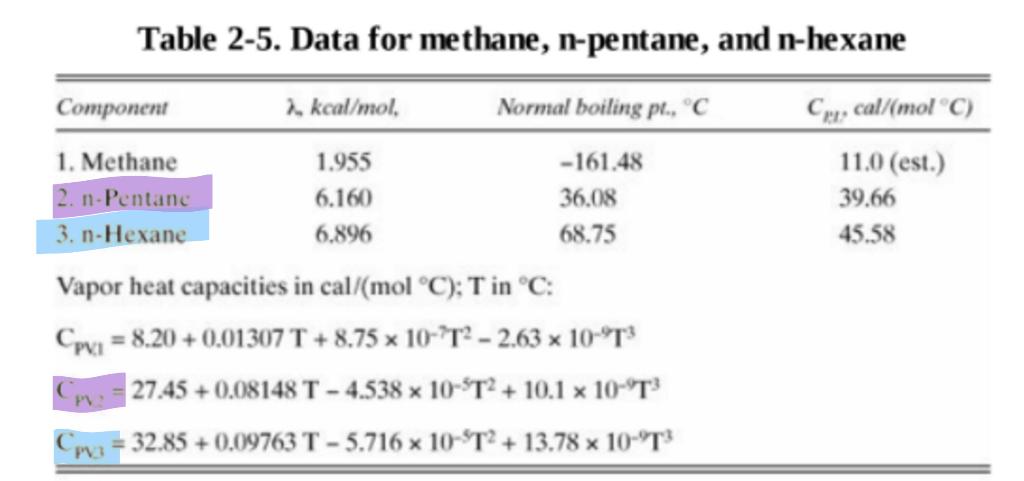
A distillation column is operating at 2 atm and is separating a feed that contains 55 mol% n-pentane and remainder is n-hexane. The feed is at 65 C and its flow rate is 1000 kmoles/hour. The distillate is 99.93 mol% n-pentane and we would like a 99.5% recovery of n-pentane at the end of the process. The system uses a total condenser, and reflux is saturated liquid with an external reflux ratio of L/D=2.8. However, there is a partial reboiler at the bottom of the column. Find the following parameters; D, B, XB, Qc, and QR. Hint: Choose reference temperature as 25 degrees C. Determine Cp for both components using data provided in Table 2-5 of Wankat. Use Cp,l and lambda values as shown in table. Other data sources include this link Table 2-5. Data for methane, n-pentane, and n-hexane Cor, cal/molC) 11.0 (est.) 39.66 45.58 Component 2. kcal/mol. Normal boiling pl., C 1. Methane 1.955 -161.48 2. n-Pentanc 6.160 36.08 3. n-Hexane 6.896 68.75 Vapor heat capacities in cal/mol C); T in C: Cpxa = 8.20 +0.01307 T + 8.75 x 10-2T2 - 2.63 x 10-T3 27.45 +0.08148 T - 4.538 x 10-T2 + 10.1 x 10-T} Co = 32.85 +0.09763 T - 5.716 x 10-T2 + 13.78 x 10-T3 PMStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


