Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I got these two questions incorrect. can explain the correct to is the one that chose In a steady-state system (i.e. systems found in a
I got these two questions incorrect. can explain the correct to is the one that chose
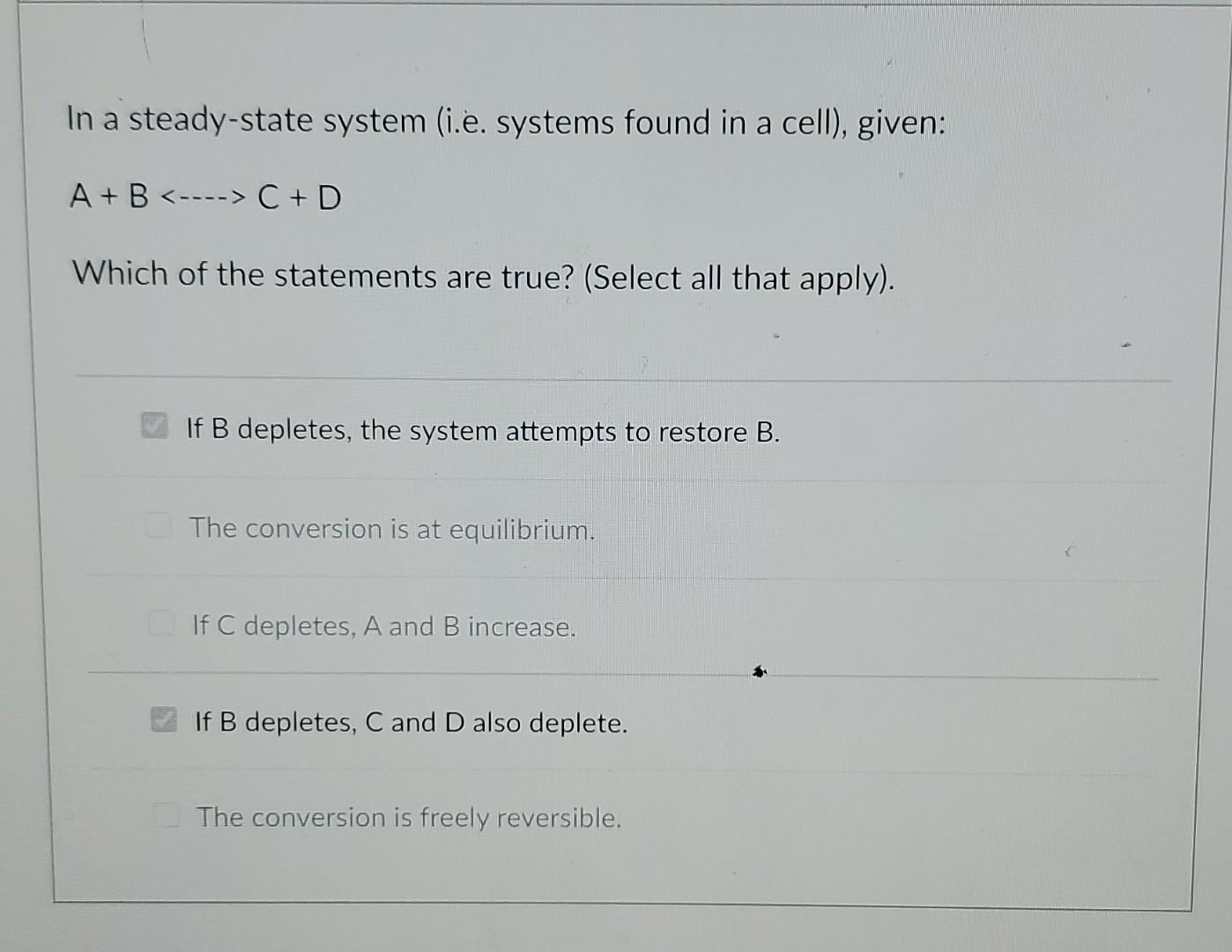
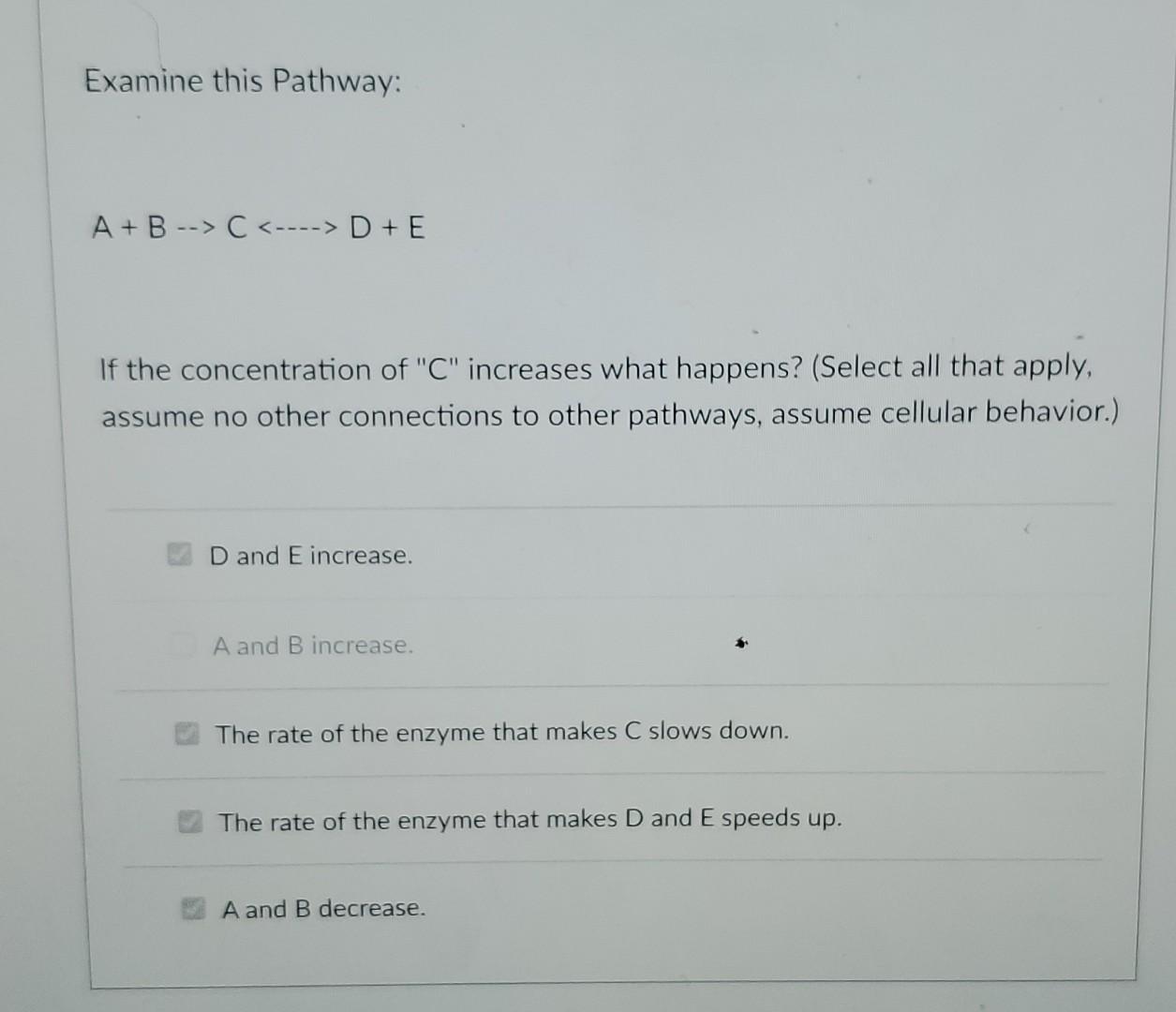
In a steady-state system (i.e. systems found in a cell), given: A + B C + D Which of the statements are true? (Select all that apply). If B depletes, the system attempts to restore B. The conversion is at equilibrium. If C depletes, A and B increase. If B depletes, C and D also deplete. The conversion is freely reversible. Examine this Pathway: A + B -->C D + E If the concentration of "C" increases what happens? (Select all that apply, assume no other connections to other pathways, assume cellular behavior.) D and E increase. A and B increase. The rate of the enzyme that makes C slows down. The rate of the enzyme that makes D and E speeds up. A and B decreaseStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


