Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I HAVE ASKED THIS QUESTION FOR SEVERAL TIMES PLEASE ANSWER IF YOU KNOW THE CORRECT SOLUTION ON THE PAPER. PLEASE SOLVE AND EXPLAIN CLEARLY. 40kmol
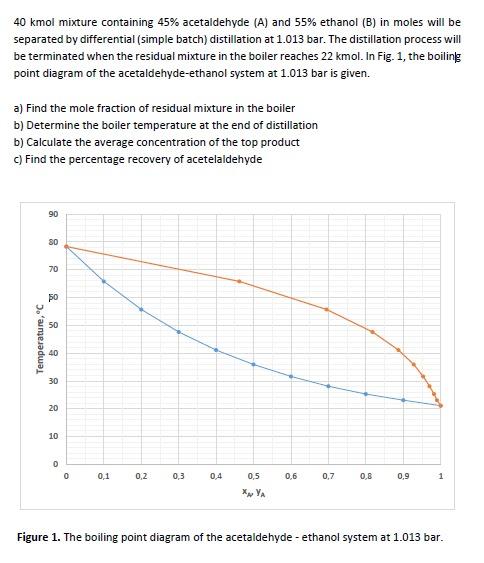
I HAVE ASKED THIS QUESTION FOR SEVERAL TIMES PLEASE ANSWER IF YOU KNOW THE CORRECT SOLUTION ON THE PAPER. PLEASE SOLVE AND EXPLAIN CLEARLY.
40kmol mixture containing 45% acetaldehyde (A) and 55% ethanol (B) in moles will be separated by differential (simple batch) distillation at 1.013 bar. The distillation process will be terminated when the residual mixture in the boiler reaches 22kmol. In Fig. 1, the boiling point diagram of the acetaldehyde-ethanol system at 1.013 bar is given. a) Find the mole fraction of residual mixture in the boiler b) Determine the boiler temperature at the end of distillation b) Calculate the average concentration of the top product c) Find the percentage recovery of acetelaldehyde Figure 1. The boiling point diagram of the acetaldehyde - ethanol system at 1.013 bar. 40kmol mixture containing 45% acetaldehyde (A) and 55% ethanol (B) in moles will be separated by differential (simple batch) distillation at 1.013 bar. The distillation process will be terminated when the residual mixture in the boiler reaches 22kmol. In Fig. 1, the boiling point diagram of the acetaldehyde-ethanol system at 1.013 bar is given. a) Find the mole fraction of residual mixture in the boiler b) Determine the boiler temperature at the end of distillation b) Calculate the average concentration of the top product c) Find the percentage recovery of acetelaldehyde Figure 1. The boiling point diagram of the acetaldehyde - ethanol system at 1.013 barStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


