Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I have been trying to get these answers on this two part but I cannot seem to figure it out! For part 1 I also

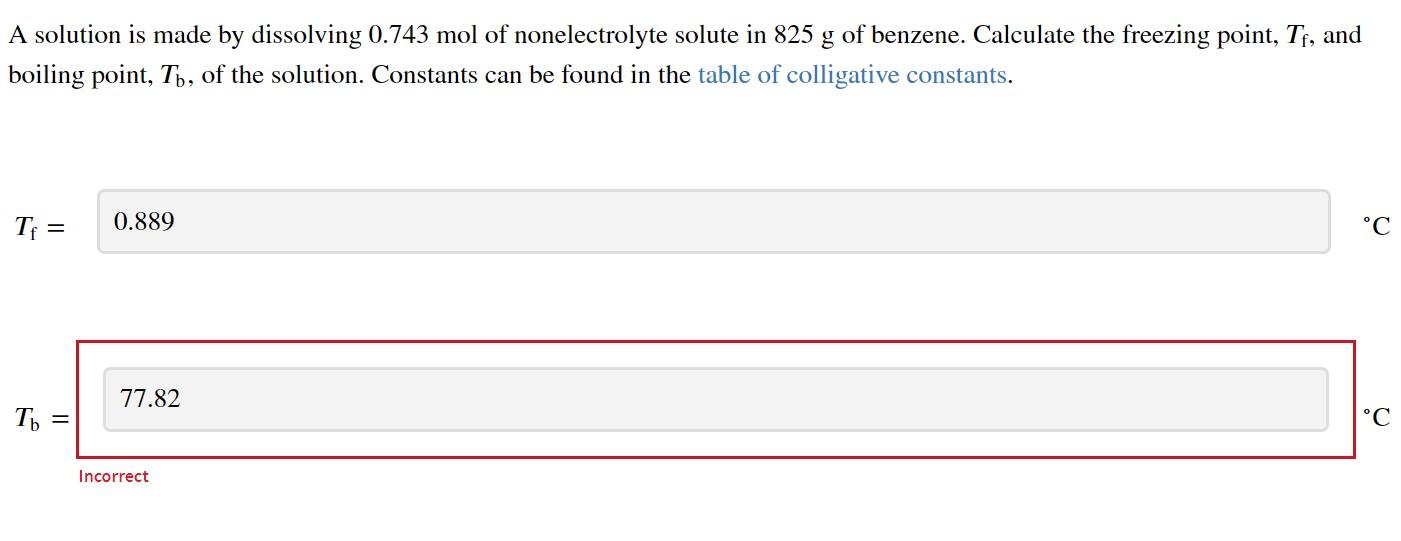 I have been trying to get these answers on this two part but I cannot seem to figure it out! For part 1 I also answered 4.23 and for the bottom I am not sure if I am just missing a sig fig...
I have been trying to get these answers on this two part but I cannot seem to figure it out! For part 1 I also answered 4.23 and for the bottom I am not sure if I am just missing a sig fig...
A chemist combined chloroform (CHCl3) and acetone (C3H6O) to create a solution where the mole fraction of chloroform, chloroform, is 0.203. The densities of chloroform and acetone are 1.48g/mL and 0.791g/mL, respectively. Calculate the molarity of the solution. Assume the volumes are additive. molarity: Calculate the molality of the solution. A solution is made by dissolving 0.743mol of nonelectrolyte solute in 825g of benzene. Calculate the freezing point, Tf, and boiling point, Tb, of the solution. Constants can be found in the table of colligative constants. Tf= Tb
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


