Question
I. In a process that produces KNO3 ? H2O (potassium nitratemonohydrate), the evaporator is fed with 2500 kg / h of a solutioncontaining 20% KNO3
I. In a process that produces KNO3 ? H2O (potassium nitratemonohydrate), the evaporator is fed with 2500 kg / h of a solutioncontaining 20% KNO3 by weight and is concentrated at a pressure of600 mmHg, obtaining an increase in boiling point of 35 ? C. Thisconcentrated solution is fed to a crystallizer that works at 115 ?F, where KNO3 ? H2O crystals are obtained, the mother liquorsolution, which contains 37.5% weight of KNO3, is recirculated tothe evaporator. Calculate the required flows, if the heat transferefficiency in the evaporator is 78%. Neglect the heat ofmixing.
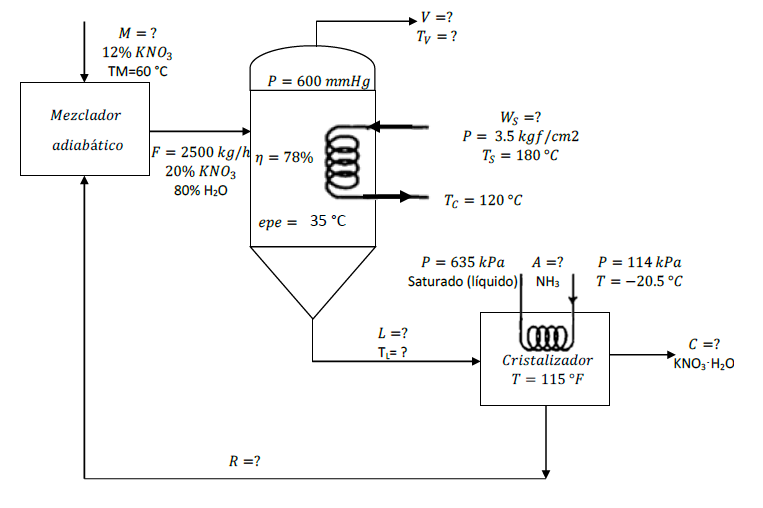
M = ? 12% KNO3 TM=60 C Mezclador adiabtico P = 600 mmHg F = 2500 kg/h n = 78% 20% KNO3 80% HO epe = 35 C R = ? V =? Ty = ? L = ? T= ? Ws =? P = 3.5 kgf/cm2 Ts = 180 C Tc = 120 C P = 635 kPa Saturado (lquido) A = ? NH3 Imm Cristalizador T = 115 F P = 114 kPa T = -20.5 C C = ? KNO3 HO
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


