Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I know its wrong but is there any way to still do it? 0. Sketch the appearance of your first derivative plot as displayed on
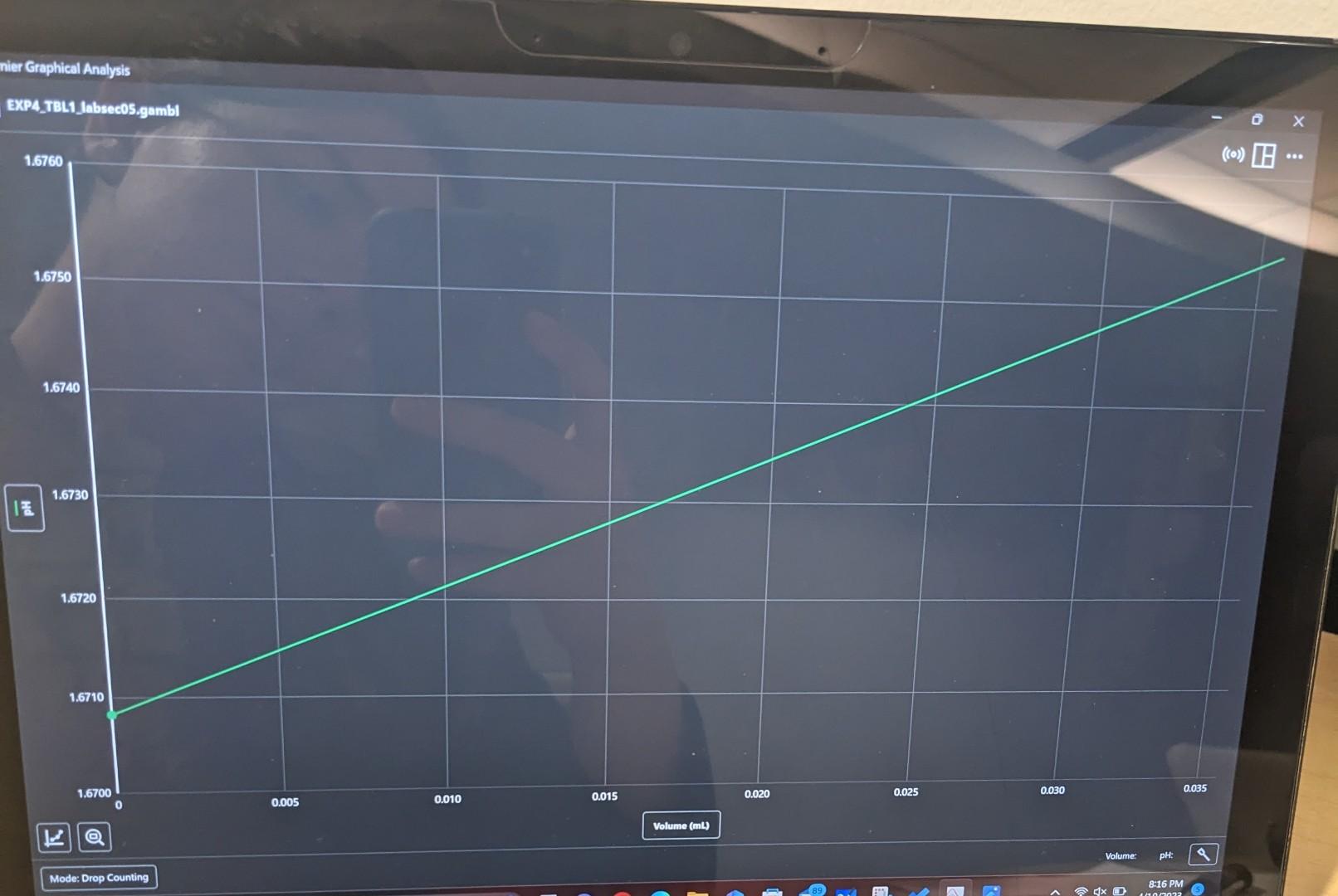
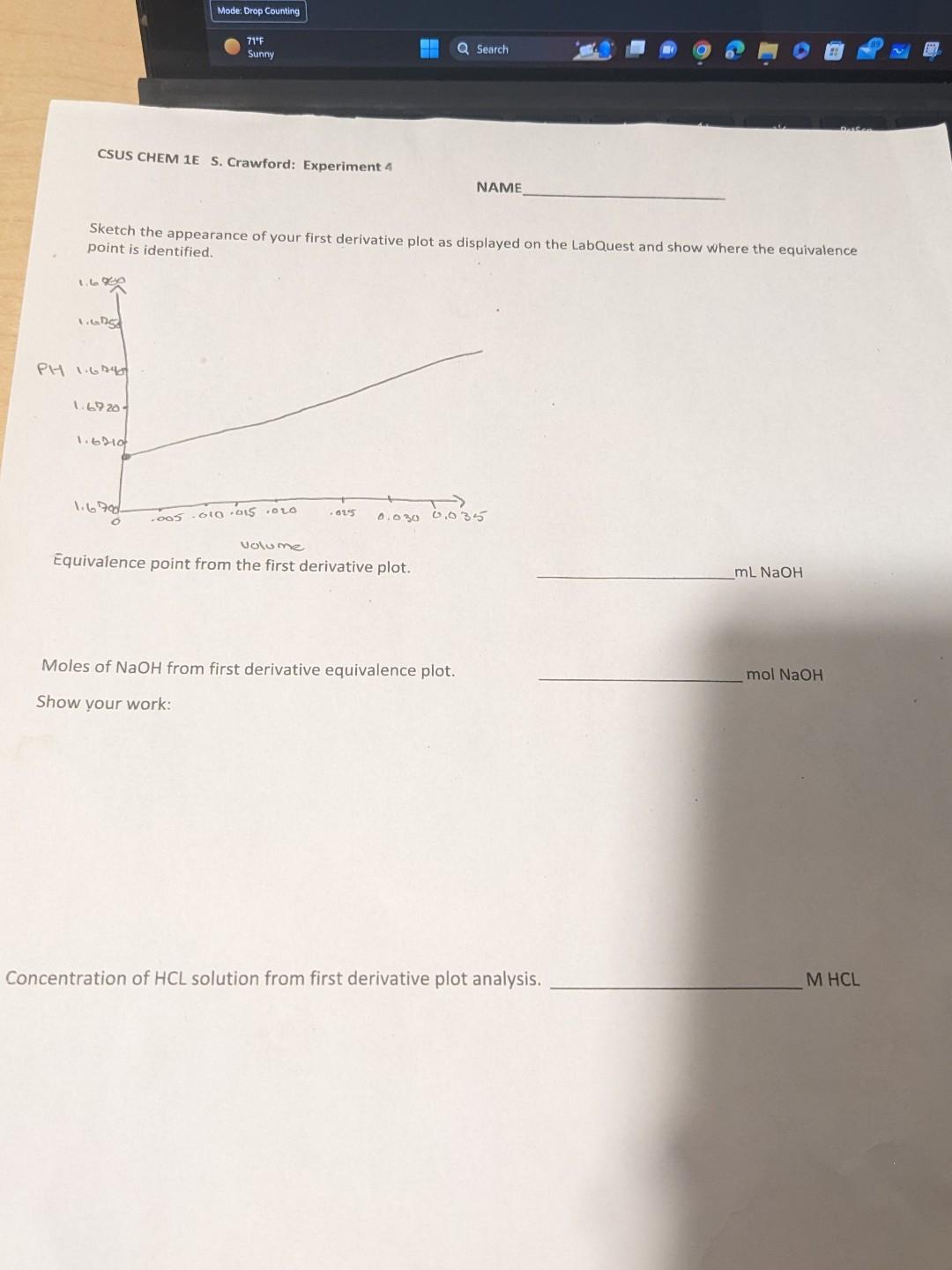

I know its wrong but is there any way to still do it?
0. Sketch the appearance of your first derivative plot as displayed on the LabQuest and show where the equivalence point is identified. Equivalence point from the first derivative plot. mLNaOH Moles of NaOH from first derivative equivalence plot. molNaOH Show your work: Concentration of HCL solution from first derivative plot analysis. MHCL A. Drop Counter Calibration: LABQUEST UNIT NUMBER 2 Volume of NaOH dispensed in calibration 9mL Drops per milliliter from LABQUEST U.CM4 ol. 8. Titration Data: 20mL1000mL12=0.02L File Name Trial 1 : Table File Name Trial 1: Expy - TQll Moles NaOH added to reach equivalence point 1.9998103molStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


