Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i Mg3(g) ii A15+ (g) Mg4* (g) + e Al6+(g) + e 6 The graph shows a sketch of log 10 ionisation energy against
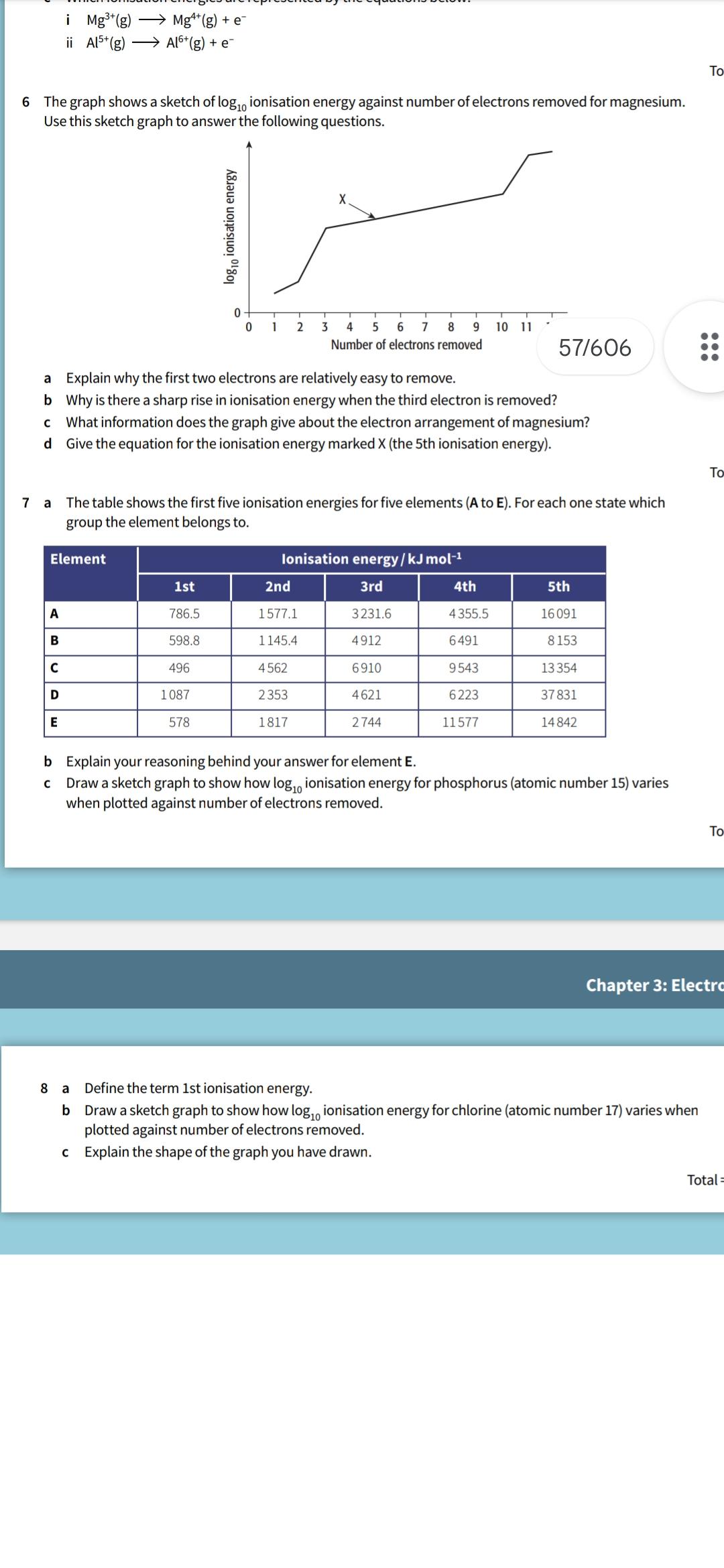
i Mg3(g) ii A15+ (g) Mg4* (g) + e Al6+(g) + e 6 The graph shows a sketch of log 10 ionisation energy against number of electrons removed for magnesium. Use this sketch graph to answer the following questions. log 10 ionisation energy X 0 1 2 3 4 5 6 7 8 9 Number of electrons removed 10 11 57/606 a Explain why the first two electrons are relatively easy to remove. b Why is there a sharp rise in ionisation energy when the third electron is removed? C What information does the graph give about the electron arrangement of magnesium? d Give the equation for the ionisation energy marked X (the 5th ionisation energy). 7 a The table shows the first five ionisation energies for five elements (A to E). For each one state which group the element belongs to. Element Ionisation energy/kJ mol-1 1st 2nd 3rd 4th 5th A 786.5 1577.1 3231.6 4355.5 16091 B 598.8 1145.4 4912 6491 8153 C 496 4562 6910 9543 13354 D 1087 2353 4621 6223 37831 E 578 1817 2744 11577 14842 b Explain your reasoning behind your answer for element E. Draw a sketch graph to show how log ionisation energy for phosphorus (atomic number 15) varies when plotted against number of electrons removed. To Chapter 3: Electro 8 a Define the term 1st ionisation energy. b Draw a sketch graph to show how log ionisation energy for chlorine (atomic number 17) varies when plotted against number of electrons removed. c Explain the shape of the graph you have drawn. Total = To To
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


