Question: I need a explanation for a number one and two. Thank you! This week you will be asked to make 20mL of a 10%mass/ volume
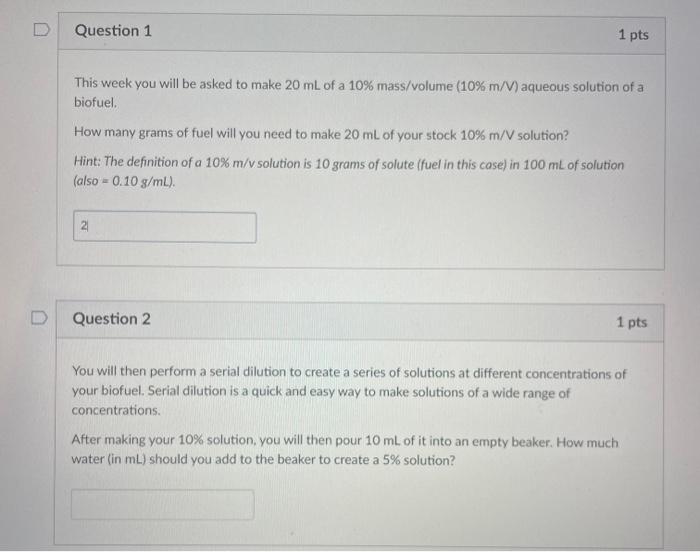
This week you will be asked to make 20mL of a 10%mass/ volume (10%m/V) aqueous solution of a biofuel. How many grams of fuel will you need to make 20mL of your stock 10%m/V solution? Hint: The definition of a 10%m/v solution is 10 grams of solute (fuel in this case) in 100mL of solution ( also =0.10g/mL). Question 2 1 pts You will then perform a serial dilution to create a series of solutions at different concentrations of your biofuel. Serial dilution is a quick and easy way to make solutions of a wide range of concentrations. After making your 10% solution, you will then pour 10mL of it into an empty beaker. How much water (in mL ) should you add to the beaker to create a 5% solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


