Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need a little assistance. I'm not really sure how to do this, but I do know it's incorrect, Question 11.b of 20 Submit Consider
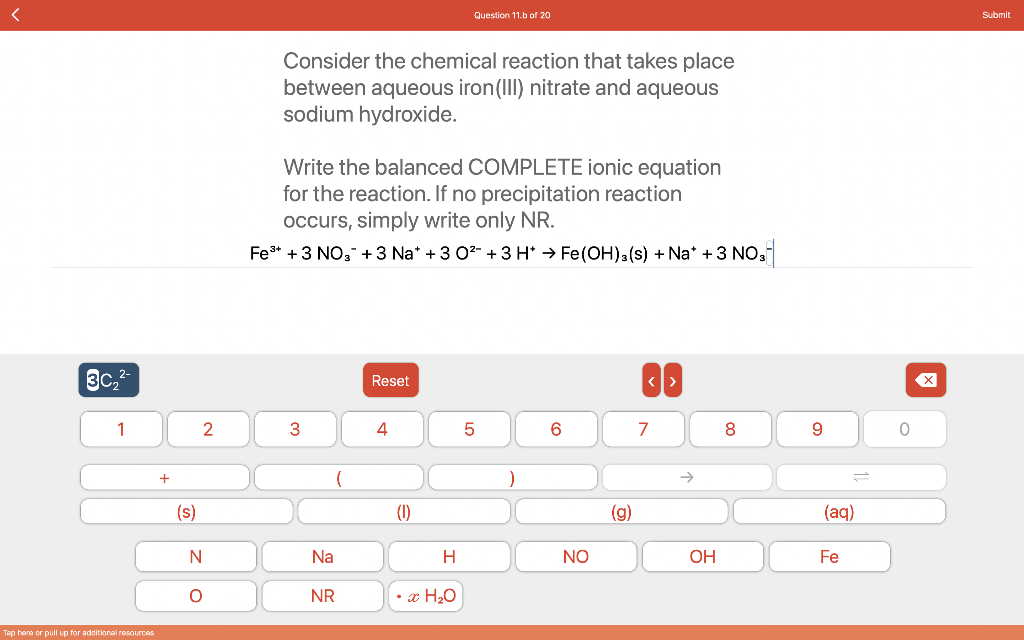
I need a little assistance. I'm not really sure how to do this, but I do know it's incorrect,
Question 11.b of 20 Submit Consider the chemical reaction that takes place between aqueous iron (III) nitrate and aqueous sodium hydroxide. Write the balanced COMPLETE ionic equation for the reaction. If no precipitation reaction occurs, simply write only NR. Fe3* +3 NO3 +3 Na* +302- +3H* Fe(OH)3(s) + Na* +3 NO 30,2 Reset x 1 2. 3 + 4 5 6 7 8 9 + ( (s) (0) (g) (aq) N Na H NO OH Fe O O NR * H2O Tap here or pull up for additional resourcesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


