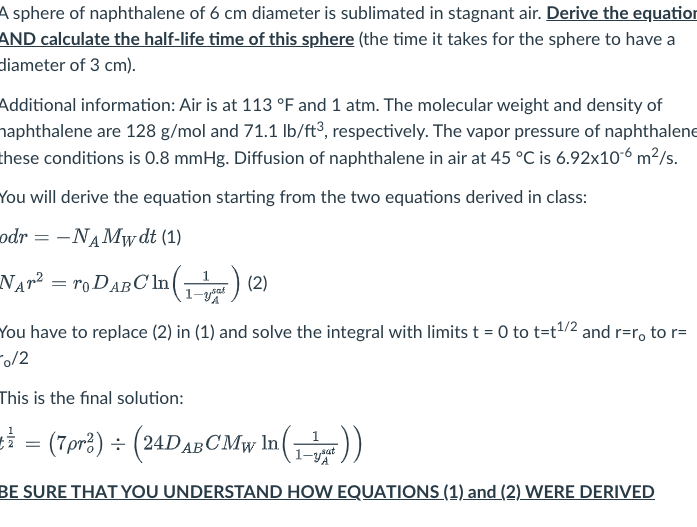
Question
I need a written solution not typed. I don't understand typed text. Please follow the general formula. N a is the flux it can be
I need a written solution not typed.
I don't understand typed text.
Please follow the general formula.
Na is the flux it can be related from Ja. Na is not avogadro's number they got it wrong on the last try. (dr) = delta radius Mw is molecular weight. dt is delate time. I need to start from the basic flux equation. Please need help with this problem
I know that Ca = yaC that is how I sub delta(ya) for where the concentration should go. Please solve this formula using this set up. Thank you
Na(flux) = (Na +NB)ya - DABC(dya/dr)
This is the general formula we need to solve with integration
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


