Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need everything answered please. its an entire experiment Pelab-plos 14 Data -p107, Yos (B+D) only. Page 103 8110-112 Post Lab-1,3,4 Date Revised: Summer 2020
i need everything answered please. its an entire experiment 
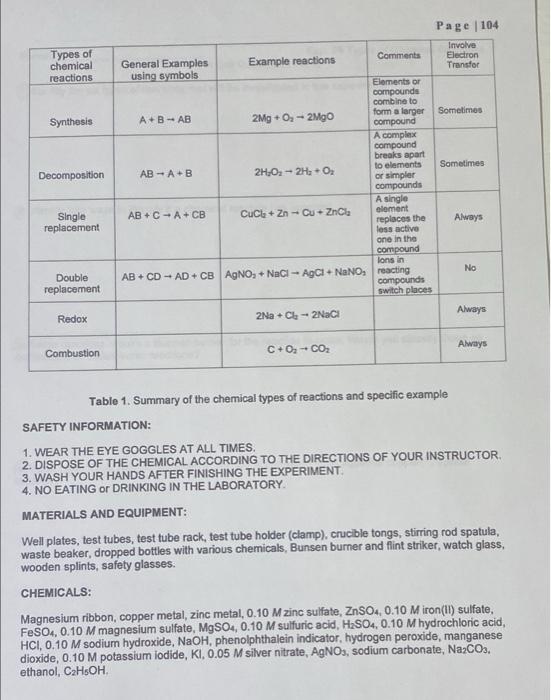


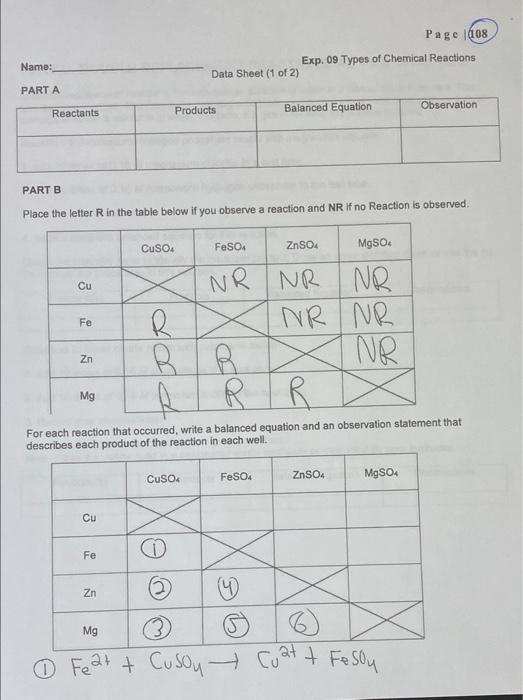


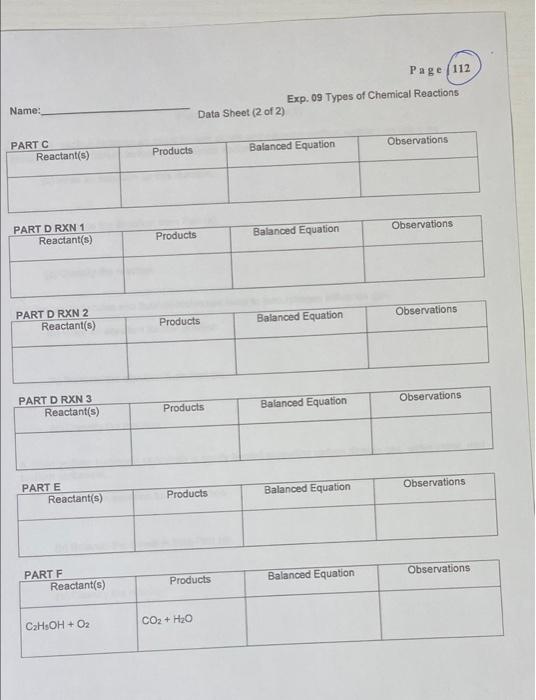

Pelab-plos 14 Data -p107, Yos (B+D) only. Page 103 8110-112 Post Lab-1,3,4 Date Revised: Summer 2020 Experiment 09 Types of Chemical Reactions LEARNING OBJECTIVES: The students will be able: 1. to make observations and classify the reaction. 2. to identify the type of reaction and predict the product(s). BACKGROUND: A chemical reaction is a process that is usually characterized by a chemical change in which the starting materials (reactants) are different from the products. Chemical reactions tend to involve the motion of electrons leading to the breaking the bonds and forming of new bonds. There are several different types of chemical reactions and more than one way of classifying them. Several observations can be made during chemical reactions: a. heat evolution b. light c. change in smell d. change in color e. gas evolution f. precipitation Often, a combination of the above observations can be observed in synergy, e.g. the reaction can give off gas and also release heat. A reaction that release heat is called exothermic and the one that absorbs heat is called endothermic. In general, a reaction that releases heat and gives off gas happens at room temperature. These are the four most common types of chemical reactions: A. Synthesis or combination B. Decomposition C. Single replacement D. Double replacement or metathesis In addition, there are two more types of reactions known as neutralization (double replacement reactions between acids and bases) and oxidation-reduction reactions. Combustion where carbon is oxidized by oxygen to produce carbon dioxide is a typical combination reaction. Page 104 Involve Electron Transfor Sometimes Sometimes Always No Always Always Types of chemical reactions Comments General Examples Example reactions using symbols Elements or compounds combine to form a larger compound Synthesis A+B-AB 2Mg + O-2MgO A complex compound breaks apart to elements or simpler compounds Decomposition AB A B 2HO-2H + O AB+C A+CB CuCl + Zn-Cu + ZnCl Single replacement A single element replaces the less active one in the compound lons in AB+CD-AD+ CB AgNO, + NaCl-AgCl + NaNO reacting Double replacement compounds switch places Redox 2Na+C-2NaCl Combustion C+0 - CO Table 1. Summary of the chemical types of reactions and specific example SAFETY INFORMATION: 1. WEAR THE EYE GOGGLES AT ALL TIMES. 2. DISPOSE OF THE CHEMICAL ACCORDING TO THE DIRECTIONS OF YOUR INSTRUCTOR. 3. WASH YOUR HANDS AFTER FINISHING THE EXPERIMENT. 4. NO EATING or DRINKING IN THE LABORATORY. MATERIALS AND EQUIPMENT: Well plates, test tubes, test tube rack, test tube holder (clamp), crucible tongs, stirring rod spatula, waste beaker, dropped bottles with various chemicals, Bunsen burner and flint striker, watch glass, wooden splints, safety glasses. CHEMICALS: Magnesium ribbon, copper metal, zinc metal, 0.10 M zinc sulfate, ZnSO4, 0.10 M iron(II) sulfate, FeSO4, 0.10 M magnesium sulfate, MgSO4, 0.10 M sulfuric acid, HSO4, 0.10 M hydrochloric acid, HCI, 0.10 M sodium hydroxide, NaOH, phenolphthalein indicator, hydrogen peroxide, manganese dioxide, 0.10 M potassium iodide, KI, 0.05 M silver nitrate, AgNO3, sodium carbonate, NaCO3. ethanol, CH5OH. Page 105 Name: Exp. 09 Types of Chemical Reactions Pre Lab Questions (1 of 1) Identify the six types of reactions that will be investigated in this experiment. Identify four ways to identify if a chemical reaction has occurred. 3 Some reactions require a Bunsen burner for the reaction to occur. Would you classify these as exothermic or endothermic reactions? 4) What type of reaction occurs when a zinc rod is immersed in an aqueous copper(II) sulfate solution? PART B. SINGLE REPLACEMENT: METAL REACTIVITIES. PROCEDURE: CuSO4 FeSO ZnSO4 MgSO 500 OO 0000 OOOO 0000 OOO OOOO 1. Obtain a 4 x 6 well plate. 2. Add a few drops of the solutions to the vertical wells as described on the figure above. 3. Place a small piece of the different metals in each of the solutions. 4. After 5-10 minutes, record your observations. Cu Fe Zn Page 107 Mg Page 108 Exp. 09 Types of Chemical Reactions Reactants Products Observation Balanced Equation. PART B Place the letter R in the table below if you observe a reaction and NR if no Reaction is observed. CuSO4 FeSO4 ZnSO4 MgSO4 NR NR NR Cu Fe R NR NR NR BB Zn Mg R R For each reaction that occurred, write a balanced equation and an observation statement that describes each product of the reaction in each well. CuSO4 FeSO4 ZnSO4 MgSO4 Cu C Fe Zn Mg 3 Fe2+ + Cusoy Cat + Fesou Name: PART A 1 Data Sheet (1 of 2) Page 110 PART D. DOUBLE REPLACEMENT REACTIONS: AB + CD AD + CB In double replacement reactions, the substances dissolve in water and dissociate to form ions. The ions are free to move. The ions in the reacting substances will switch places and a precipitate, a solid compound which is insoluble in water, will form. If a gas is formed, you will see bubbles. Reaction 1: Ammonium hydrogen phosphate reacts with magnesium chloride. 1. Add 10 drops of 0.10 M magnesium chloride to a test tube. 2. Add 10 drops of 0.10 M Ammonium hydrogen phosphate to the test tube. 3. Record your observations. 4. Write a complete balanced equation for this reaction. Check the solubility table to identify the insoluble product. Draw an arrow pointing down or use the (s) To indicate the precipitate in this reaction Reactant(s) Products Balanced Equation Observations Reaction 2: Silver nitrate reacts with potassium iodide 1. Add 10 drops of 0.10 M silver nitrate to a spot plate. 2. Add 10 drops of 0.10 M potassium iodide to the spot plate 3. Record your observations. 4. Write a complete balanced equation for this reaction. Note: Place the yellow solid in a designated waste container as instructed by your instructor Reactant(s) Products Balanced Equation Observations Page 111 Reaction 3: Sodium hydrogen carbonate (NaHCO) reacts with hydrochloric acid 1. Place about 0.5 gram (tip of spatula) of sodium hydrogen carbonate into a test tube. 2. Add 10 drops of 0.10 M HCI to the test tube. 3. Test the gas that is being given off by placing a glowing splint into the tube. 4. Record your observations. 5. Write a complete balanced equation for this reaction. Reactant(s) Products Balanced Equation Observation PART E. NEUTRALIZATION REACTION. 1. In a small test tube, place 10 drops of 0.10 M of HCI. 2. Add 1 drop of Phenolphthalein indicator to the test tube. 3. Observe the color. 4. Using a dropper, add 0.10 M of NaOH solution drop by drop until you observe a color change. Count and record the number of drops required. 5. Record your observations. Reactant(s) Products Balanced Equation Observations PART F: COMPLETE COMBUSTION. INSTRUCTOR DEMO! Reaction: The combustion of ethanol CHIOH + O2 CO2 + H2O 1. Place 5-10 drops of ethanol (CH5OH or CH3CHOH) onto a watch glass. 2. Light a wooden splint and place it near the ethanol. 3. Record your observations. 4. Write a complete balanced equation for this reaction. Reactant(s) Products Balanced Equation CzHOH + Oz CO + HO Observation Name: PART C Reactant(s) PART D RXN 1 Reactant(s) PART D RXN 2 Reactant(s) PART D RXN 3 Reactant(s) PART E Reactant(s) Reactant(s) PART F CHOH+Oz Data Sheet (2 of 2) Products Products Products Products Products Products CO + HO Page 112 Exp. 09 Types of Chemical Reactions Observations Observations Observations Observations Observations Observations Balanced Equation Balanced Equation Balanced Equation Balanced Equation Balanced Equation Balanced Equation Page 114 Name: Exp. 09 Types of Chemical Reactions Post Lab Questions (2 of 2) For the reaction between aqueous barium nitrate and aqueous sodium sulfate producing the precipitate (solid) barium sulfate and aqueous sodium nitrate (a) Write an equation for this reaction. (b) Balance the equation from Part (a). (c) Classify the reaction. (4) Nitrogen molecules and fluorine molecules react to form nitrogen trifluoride gas. Nitro (a) Write an equation for this reaction. (b) Balance the equation from Part (a). (c) Classify the reaction. 5. Solid sodium hydrogen carbonate is gently heated producing carbon dioxide gas, water vapor, and sodium carbonate. (a) Write an equation for this reaction. (b) Balance the equation from Part (a). (c) Classify the reaction 








Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


