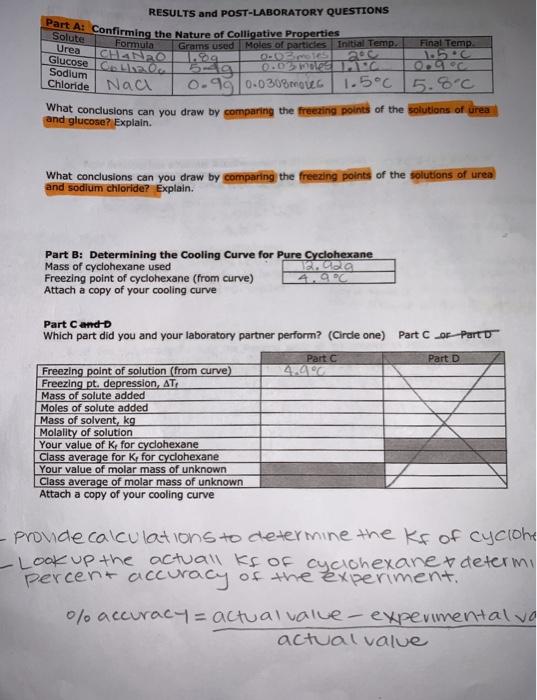
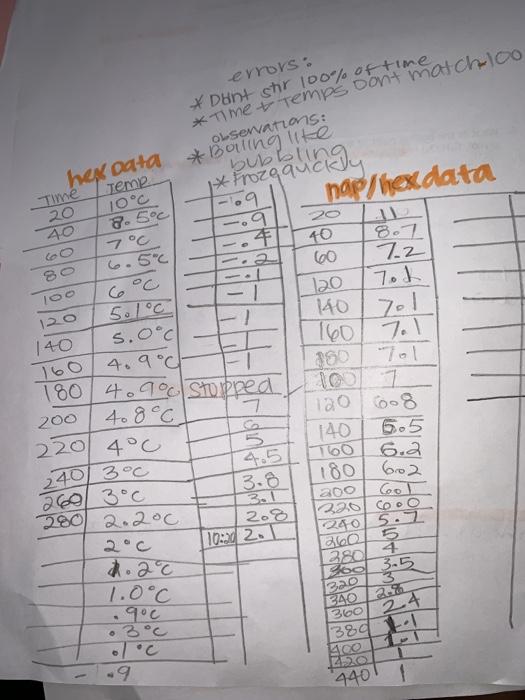
I need help filling out the table for Part C and drawing 2 cooling curves 1. for hexane data 2. for naphthelene and hexane data. please!!
Part C: Determining the K, for Cyclohexane Chemicals Used Materials Used Naphthalene ( CH) Cooling curve apparatus (from Part B) NaCl Spatula Ice Analytical balance Stopwatch Half the class, working in pairs Measure approximately 0.30 grams of naphthalene (record the actual mass used) and transfer into the test tube filled with cyclohexane that you used in Part B. Make sure the naphthalene is completely dissolved before proceeding to the next section. Add ice and salt to the 400-ml beaker as needed. Determine the freezing point of the solution in the same way you did for the pure solvent. Again, be sure to collect data for at least 10 minutes. Dispose of the cyclohexane solution according to your instructor's directions. Part A: Confirming the Nature of Colligative Properties RESULTS and POST-LABORATORY QUESTIONS Formula Final Temp Grams used Moles of particles Initial Temp. CHANZO. 105 b9 ONDE TEEN Chloride Nac 0.95 0.0308motto 1.5C 5.8C What conclusions can you draw by comparing the freezing points of the solutions of urea and glucose? Explain. Solute Urea Glucose Lika Sodium 200 What conclusions can you draw by comparing the freezing points of the solutions of urea and sodium chloride? Explain. Part B: Determining the Cooling Curve for Pure Cyclohexane Mass of cyclohexane used TECa Freezing point of cyclohexane (from curve) 4.90 Attach a copy of your cooling curve Part Cor Part Part D Part Cando Which part did you and your laboratory partner perform? (Cirde one) Part C Freezing point of solution (from curve) Freezing pt. depression, AT Mass of solute added Moles of solute added Mass of solvent kg Molality of solution Your value of K for cyclohexane Class average for K, for cyclohexane Your value of molar mass of unknown Class average of molar mass of unknown Attach a copy of your cooling curve pronde calculations to determine the kf of cyclone -Look up the actuall kf of cyclohexane determy percent accuracy of the experiment." 0% accuracy = actual value- experimentalvo actual value bubblingu errors: * Dint str 100% of time *Time & Temps don't match 100 OLservations: * Boiling like 1* Frozga -09 nap/hexdata O hex data Time Temp 20 40 8.5C 7C 6.5C 4 2 loo zo! 120 140 5.1C 5.0c 4.9c 160 40 600 7.2 120 Tok 140 160 Tol 350 Tol 7 120 140 6.2 180 602 200 320 cooo 7 180 4.90 Stopped 408C. 220 4C 200 ube 6.5 2403C 280 2.200 3.6 3.1 208 10:20 20 240 5.7 260 5 4 od 3.5 380! .20c 1.0C 9c 3C Bao 340 250 360 24 380 L 22 440 9 1