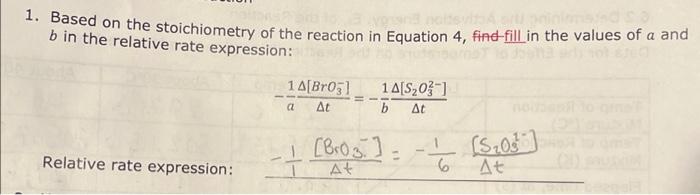I need help finding
Rate constant, k
ln k (no units)
1/T (K^-1)
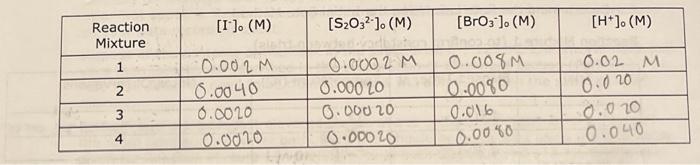
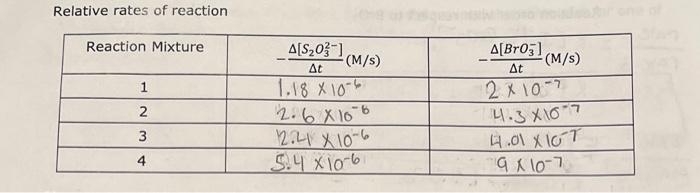
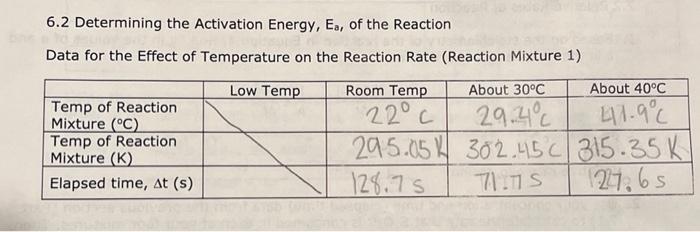
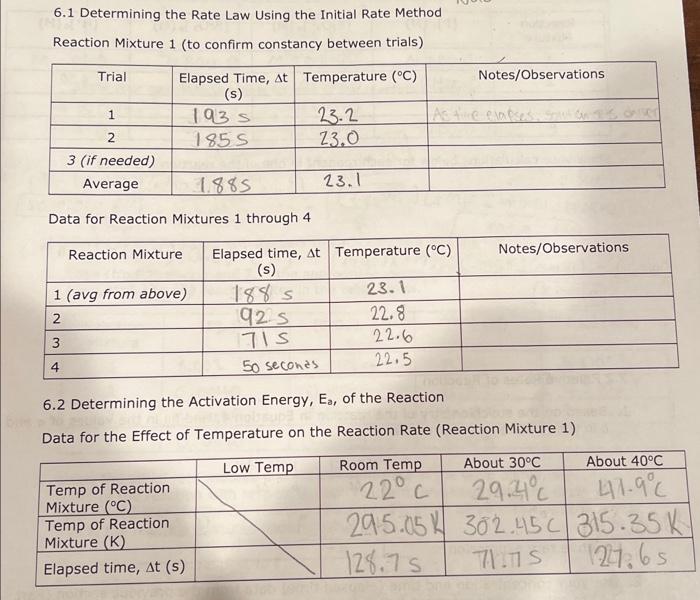
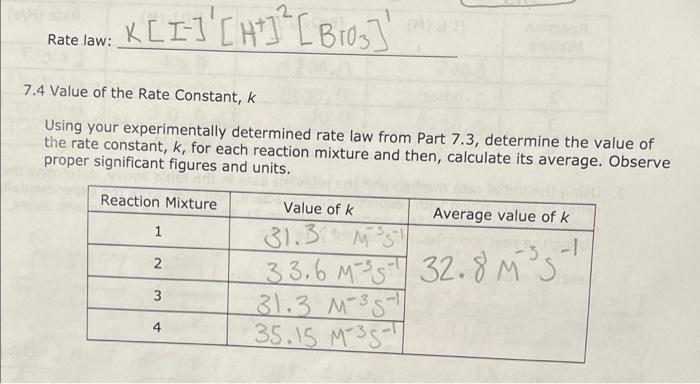
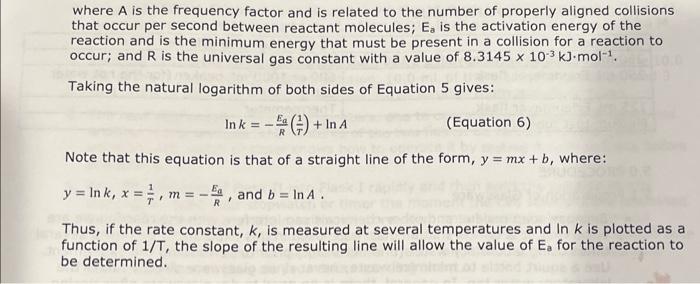
7.5 Determining the Activation Energy, E0, for the Reaction 1. Determine the value of the rate constant, k, for each temperature below and give the appropriate units. Show your calculations for one of the reaction temperatures below: 11T[BrO3]=61t[S2O32] 1About30C[71.7][0.008]=61[71.7][0.002]1.12104 11 \begin{tabular}{|c|c|c|c|c|} \hline ReactionMixture & {[I]0(M)} & {[S2O32]0(M)} & {[BrO3]0(M)} & {[H+]0(M)} \\ \hline 1 & 0.002M & 0.0002M & 0.008M & 0.02 \\ \hline 2 & 0.0040 & 0.00020 & 0.0080 & 0.020 \\ \hline 3 & 0.0020 & 0.00020 & 0.016 & 0.020 \\ \hline 4 & 0.0020 & 0.00020 & 0.0040 & 0.040 \\ \hline \end{tabular} Relative rates of reaction 6.2 Determining the Activation Energy, Ea, of the Reaction Data for the Effect of Temperature on the Reaction Rate (Reaction Mixture 1) 6.1 Determining the Rate Law Using the Initial Rate Method Reaction Mixture 1 (to confirm constancy between trials) Data for Reaction Mixtures 1 through 4 6.2 Determining the Activation Energy, Ea, of the Reaction Data for the Effect of Temperature on the Reaction Rate (Reaction Mixture 1) .4 Value of the Rate Constant, k Using your experimentally determined rate law from Part 7.3, determine the value of the rate constant, k, for each reaction mixture and then, calculate its average. Observe proper significant figures and units. 1. Based on the stoichiometry of the reaction in Equation 4 , find fill in the values of a and b in the relative rate expression: t1[BrO3]=b1t[S2O32]Relativerateexpression:11t[BrO3]=61t[S2O31] where A is the frequency factor and is related to the number of properly aligned collisions that occur per second between reactant molecules; Ea is the activation energy of the reaction and is the minimum energy that must be present in a collision for a reaction to occur; and R is the universal gas constant with a value of 8.3145103kJmol1. Taking the natural logarithm of both sides of Equation 5 gives: lnk=REa(1)+lnA (Equation 6) Note that this equation is that of a straight line of the form, y=mx+b, where: y=lnk,x=T1,m=REa, and b=lnA Thus, if the rate constant, k, is measured at several temperatures and lnk is plotted as a function of 1/T, the slope of the resulting line will allow the value of Ea for the reaction to be determined. 6I(aq)+BrO3(aq)+6H+(aq)3I2(aq)+Br(aq)+3H2O(I)slow(clockreaction)3I2(aq)+6S2O32(aq)6I(aq)+3S4O62(aq)fast Overall reaction: BrO3(aq)+6S2O32(aq)+6H+(aq)Br(aq)+3H2O(I)+3S4O62-(aq)(Equation4) Note that since the 'clock' reaction is relatively fast, the I2(aq) will be consumed by the clock reaction as quickly as it can be produced by the reaction of interest thus holding the I2 concentration at a very low value close to zero. Only after the S2O32 has been entirely consumed will the concentration of I2 start to increase. After the S2O32 - has been consumed, the concentration of I2 will increase rapidly allowing the I2 to react with a starch indicator resulting in the solution to acquire a deep blue color. In this experiment, you will measure the time required for the solution to turn blue. This is essentially the amount of time required for all the S2O32 to be reacted. A stoichiometric calculation can be used to determine the number of moles of BrO3 reacted at the point in time when all the S2O32 - has been consumed. By dividing the number of moles of BrO3 - reacted by the total volume of the reaction mixture we can obtain the change in the BrO3 concentration. We can obtain the reaction rate by dividing this change in concentration by the amount of time required for the blue color to appear (t) a. Measure the time required for the solution to turn blue. (all S2O3 - reacted) b. Calculate moles BrO3 reacted c. Divide moles BrO3 by total volume of reaction mixture =[BrO3] d. Reaction rate =[BrO3]divided by a. =[BrO3]/(t) 1.2.2 Determining the Activation Energy of the Reaction The value of the rate constant, k, measured previously is dependent upon the temperature at which the reaction occurs according to the Arrhenius Equation: k=AeEa/RT (Equation 5) 7.5 Determining the Activation Energy, E0, for the Reaction 1. Determine the value of the rate constant, k, for each temperature below and give the appropriate units. Show your calculations for one of the reaction temperatures below: 11T[BrO3]=61t[S2O32] 1About30C[71.7][0.008]=61[71.7][0.002]1.12104 11 \begin{tabular}{|c|c|c|c|c|} \hline ReactionMixture & {[I]0(M)} & {[S2O32]0(M)} & {[BrO3]0(M)} & {[H+]0(M)} \\ \hline 1 & 0.002M & 0.0002M & 0.008M & 0.02 \\ \hline 2 & 0.0040 & 0.00020 & 0.0080 & 0.020 \\ \hline 3 & 0.0020 & 0.00020 & 0.016 & 0.020 \\ \hline 4 & 0.0020 & 0.00020 & 0.0040 & 0.040 \\ \hline \end{tabular} Relative rates of reaction 6.2 Determining the Activation Energy, Ea, of the Reaction Data for the Effect of Temperature on the Reaction Rate (Reaction Mixture 1) 6.1 Determining the Rate Law Using the Initial Rate Method Reaction Mixture 1 (to confirm constancy between trials) Data for Reaction Mixtures 1 through 4 6.2 Determining the Activation Energy, Ea, of the Reaction Data for the Effect of Temperature on the Reaction Rate (Reaction Mixture 1) .4 Value of the Rate Constant, k Using your experimentally determined rate law from Part 7.3, determine the value of the rate constant, k, for each reaction mixture and then, calculate its average. Observe proper significant figures and units. 1. Based on the stoichiometry of the reaction in Equation 4 , find fill in the values of a and b in the relative rate expression: t1[BrO3]=b1t[S2O32]Relativerateexpression:11t[BrO3]=61t[S2O31] where A is the frequency factor and is related to the number of properly aligned collisions that occur per second between reactant molecules; Ea is the activation energy of the reaction and is the minimum energy that must be present in a collision for a reaction to occur; and R is the universal gas constant with a value of 8.3145103kJmol1. Taking the natural logarithm of both sides of Equation 5 gives: lnk=REa(1)+lnA (Equation 6) Note that this equation is that of a straight line of the form, y=mx+b, where: y=lnk,x=T1,m=REa, and b=lnA Thus, if the rate constant, k, is measured at several temperatures and lnk is plotted as a function of 1/T, the slope of the resulting line will allow the value of Ea for the reaction to be determined. 6I(aq)+BrO3(aq)+6H+(aq)3I2(aq)+Br(aq)+3H2O(I)slow(clockreaction)3I2(aq)+6S2O32(aq)6I(aq)+3S4O62(aq)fast Overall reaction: BrO3(aq)+6S2O32(aq)+6H+(aq)Br(aq)+3H2O(I)+3S4O62-(aq)(Equation4) Note that since the 'clock' reaction is relatively fast, the I2(aq) will be consumed by the clock reaction as quickly as it can be produced by the reaction of interest thus holding the I2 concentration at a very low value close to zero. Only after the S2O32 has been entirely consumed will the concentration of I2 start to increase. After the S2O32 - has been consumed, the concentration of I2 will increase rapidly allowing the I2 to react with a starch indicator resulting in the solution to acquire a deep blue color. In this experiment, you will measure the time required for the solution to turn blue. This is essentially the amount of time required for all the S2O32 to be reacted. A stoichiometric calculation can be used to determine the number of moles of BrO3 reacted at the point in time when all the S2O32 - has been consumed. By dividing the number of moles of BrO3 - reacted by the total volume of the reaction mixture we can obtain the change in the BrO3 concentration. We can obtain the reaction rate by dividing this change in concentration by the amount of time required for the blue color to appear (t) a. Measure the time required for the solution to turn blue. (all S2O3 - reacted) b. Calculate moles BrO3 reacted c. Divide moles BrO3 by total volume of reaction mixture =[BrO3] d. Reaction rate =[BrO3]divided by a. =[BrO3]/(t) 1.2.2 Determining the Activation Energy of the Reaction The value of the rate constant, k, measured previously is dependent upon the temperature at which the reaction occurs according to the Arrhenius Equation: k=AeEa/RT (Equation 5)