i need help.
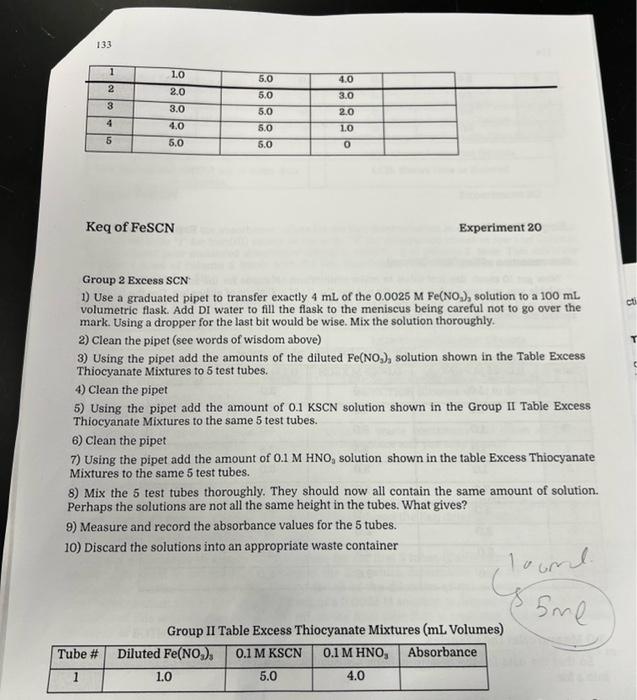
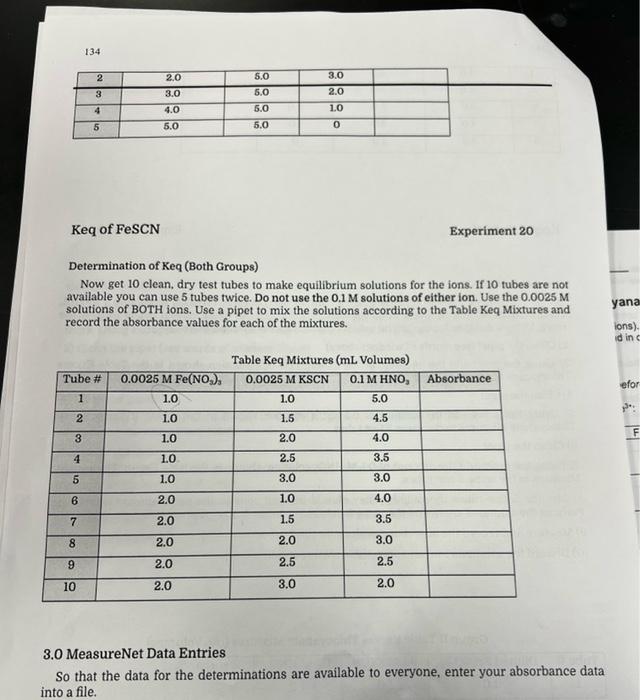
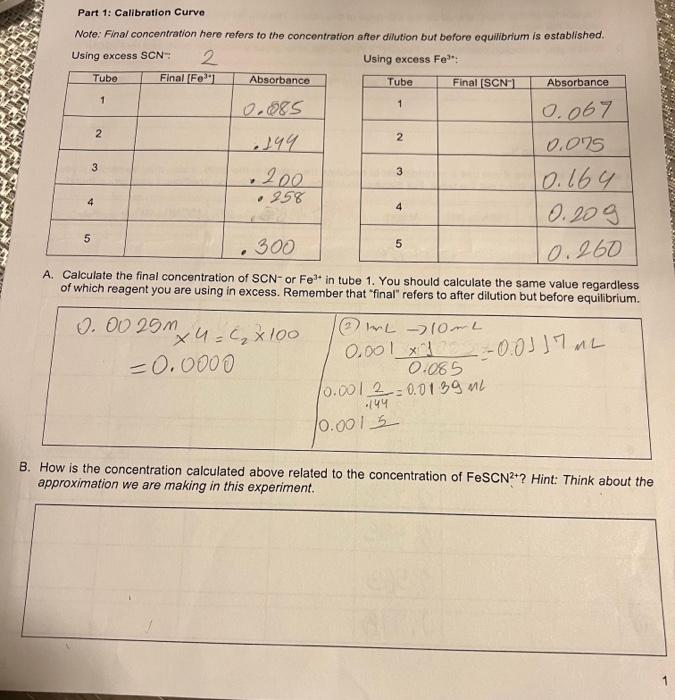
Group 2 Excess SCN 1) Use a graduated pipet to transfer exactly 4mL of the 0.0025MFe(NO2)2 solution to a 100mL volumetric flask. Add DI water to fill the flask to the meniscus being careful not to go over the mark. Using a dropper for the last bit would be wise. Mix the solution thoroughly. 2) Clean the pipet (see words of wisdom above) 3) Using the pipet add the amounts of the diluted Fe(NO2)2 solution shown in the Table Excess Thiocyanate Mixtures to 5 test tubes. 4) Clean the pipet 5) Using the pipet add the amount of 0.1KSCN solution shown in the Group II Table Excess Thiocyanate Mixtures to the same 5 test tubes. 6) Clean the pipet 7) Using the pipet add the amount of 0.1MHNO3 solution shown in the table Excess Thiocyanate Mixtures to the same 5 test tubes. 8) Mix the 5 test tubes thoroughly. They should now all contain the same amount of solution. Perhaps the solutions are not all the same height in the tubes. What gives? 9) Measure and record the absorbance values for the 5 tubes. 10) Discard the solutions into an appropriate waste container Group II Table Excess Thiocyanate Mixtures (mL Volumes) 134 Keq of FeSCN Experiment 20 Determination of Keq (Both Groups) Now get 10 clean, dry test tubes to make equilibrium solutions for the ions. If 10 tubes are not available you can use 5 tubes twice. Do not use the 0.1M solutions of either ion. Use the 0.0025M solutions of BOTH ions. Use a pipet to mix the solutions according to the Table Keq Mixtures and record the absorbance values for each of the mixtures. Table Kea Mixtures (mL. Volumes) 3.0 MeasureNet Data Entries So that the data for the determinations are available to everyone, enter your absorbance data into a file. Part 1: Calibration Curve Note: Final concentration here refers to the concentration after dillution but before equilibrium is established. Using excess SCN: Using excess Fe3? A. Calculate the final concentration of SCNor Fe3+ in tube 1. You should calculate the same value regardless of which reagent you are using in excess. Remember that "final" refers to after dilution but before equilibrium. 3. How is the concentration calculated above related to the concentration of FeSCN2+ ? Hint: Think about the approximation we are making in this experiment. C. Generate two scatter plots of absorbance versus concentration in your preferred spreadsheot program, You should plot both your group's data and the other group's data. Your scatter plots should include tho following. - A title - Laboled axes with correct units - Linear trendline - Trend line equation with its R2 value The plot for excess SCN* should be the fifth page of this report. The plot for excess Fe S3+ should be the sixth page of this report