Answered step by step
Verified Expert Solution
Question
1 Approved Answer
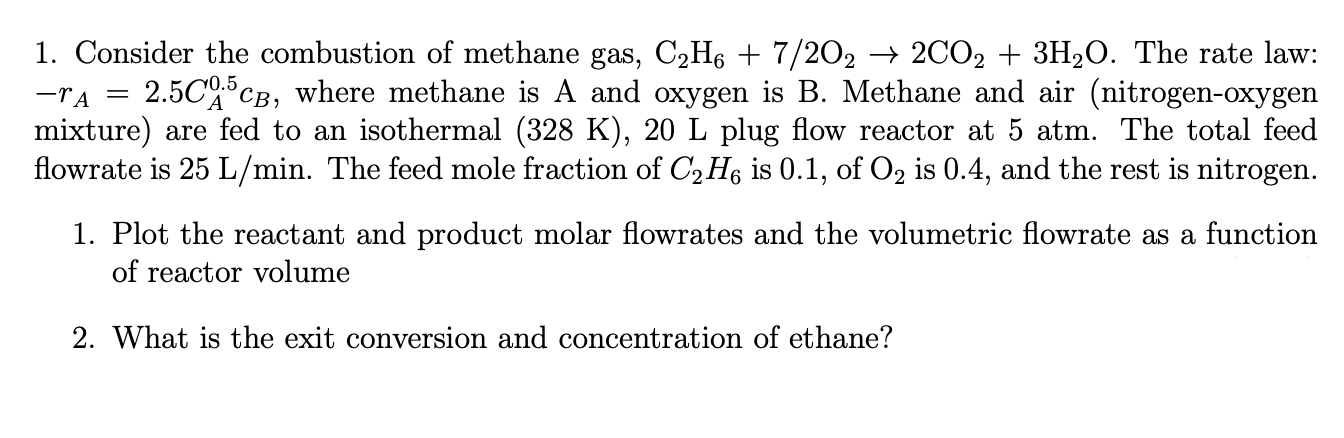
I need help on 1 and 2 of this problem. I will leave a like. Consider the combustion of methane gas, C 2 H 6
I need help on and of this problem. I will leave a like.
Consider the combustion of methane gas, The rate law:
where methane is A and oxygen is B Methane and air nitrogenoxygen
mixture are fed to an isothermal plug flow reactor at atm. The total feed
flowrate is The feed mole fraction of is of is and the rest is nitrogen.
Plot the reactant and product molar flowrates and the volumetric flowrate as a function
of reactor volume
What is the exit conversion and concentration of ethane?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


