Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help on all of them please!!!! help me out!!! Question 3 Calculate the pH of a solution that is 0.350 M in the
I need help on all of them please!!!!
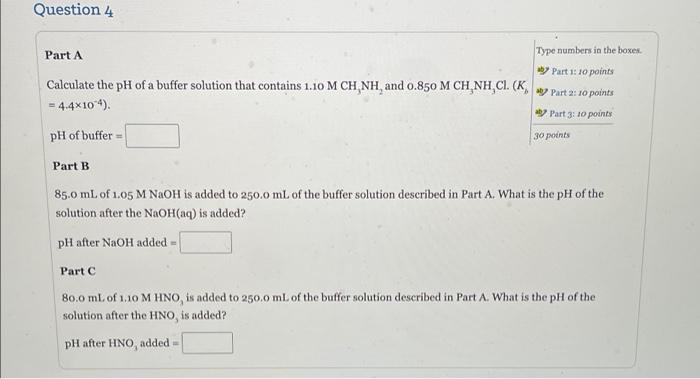
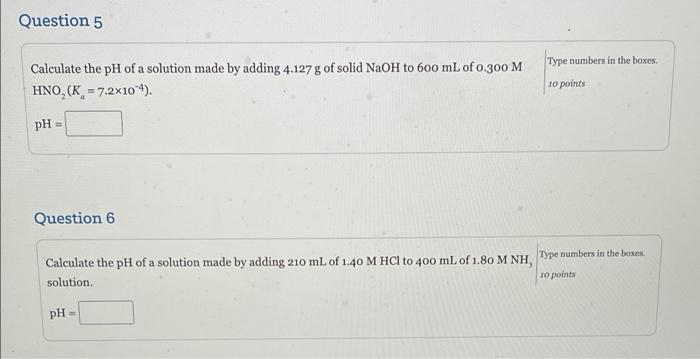
Question 3 Calculate the pH of a solution that is 0.350 M in the weak acid HA (K = 1.41x10) and 0.250 Min A. Type numbers in the boxes 10 points pH Question 4 Part A Type numbers in the boxes Part 1: 10 points Calculate the pH of a buffer solution that contains 1.10 M CH NH, and 0.850 M CH, NH,CI (K Part 2: 10 points = 4.4x104). aby Part 3: 10 points pH of buffer 30 points Part B 85.0 mL of 1.05 M NaOH is added to 250,0 mL of the buffer solution described in Part A. What is the pH of the solution after the NaOH(aq) is added? pH after NaOH added Part C 80.0 mL of 1.10 M HNO is added to 250,0 mL of the buffer solution described in Part A. What is the pH of the solution after the HNO is added? pH after HNO, added - Question 5 Type numbers in the boxes g Calculate the pH of a solution made by adding 4.127 g of solid NaOH to 600 mL of 0.300 M HNO (K = 7.2x104). 10 points pH = Question 6 Calculate the pH of a solution made by adding 210 mL of 1.40 M HCl to 400 mL of 1.80 M NH, Type numbers in the boxes. solution. 10 points pH = help me out!!! 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


