Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help on both and also this one A solution contains a hydroxide ion concentration [OH] of 1mM. What is the pH of this
i need help on both 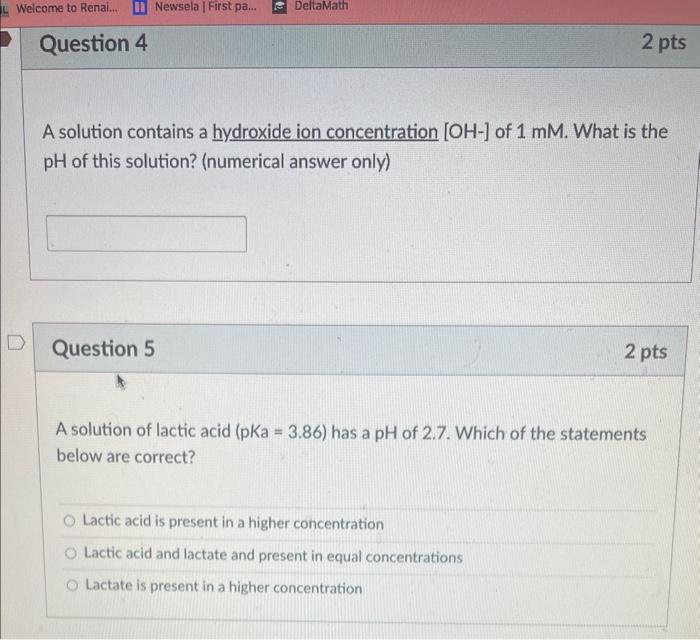 and also this one
and also this one 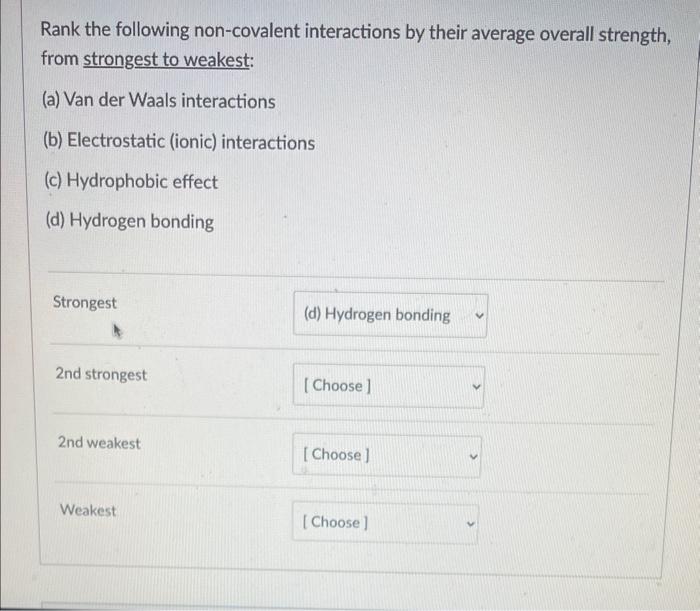 A solution contains a hydroxide ion concentration [OH] of 1mM. What is the pH of this solution? (numerical answer only) Question 5 2 pts A solution of lactic acid ( pKa=3.86 ) has a pH of 2.7. Which of the statements below are correct? Lactic acid is present in a higher concentration Lactic acid and lactate and present in equal concentrations Lactate is present in a higher concentration Rank the following non-covalent interactions by their average overall strength, from strongest to weakest: (a) Van der Waals interactions (b) Electrostatic (ionic) interactions (c) Hydrophobic effect (d) Hydrogen bonding Strongest 2nd strongest 2nd weakest Weakest
A solution contains a hydroxide ion concentration [OH] of 1mM. What is the pH of this solution? (numerical answer only) Question 5 2 pts A solution of lactic acid ( pKa=3.86 ) has a pH of 2.7. Which of the statements below are correct? Lactic acid is present in a higher concentration Lactic acid and lactate and present in equal concentrations Lactate is present in a higher concentration Rank the following non-covalent interactions by their average overall strength, from strongest to weakest: (a) Van der Waals interactions (b) Electrostatic (ionic) interactions (c) Hydrophobic effect (d) Hydrogen bonding Strongest 2nd strongest 2nd weakest Weakest
i need help on both 

and also this one

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


