Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help! p=10 T=800 P4. In this problem, you will calculate the degree of dissociation, a, and the partial pressure of product formed for
I need help!
P4. In this problem, you will calculate the degree of dissociation, a, and the partial pressure of product formed for the oxidation of sulfur dioxide under different pressure and temperature conditions. 2 SO2(g) + O2(g) + 2 SO3(g) The equilibrium constant for this reaction is 3.68 (with p = 1 bar) at 940 K. Assigned temperatures are given in an Excel file in Google drive: HW4_SO2-SO3-reaction_2022. Place your answers in this file, and hand in your calculations. a) Derive expressions for the partial pressures of each component in terms of the degree of dissociation, a, and the total pressure p. Start with 2 moles of SO2, I moles of O2, and no product. Then write the expression for the equilibrium constant. b) Determine a for p = 1.00 bar at 940 K. Then, calculate the partial pressures of all of the components. c) Calculate the equilibrium constant for the reaction the temperature you are assigned. Unless you can find a better value, use the enthalpy of the reaction calculated at 298 K. Place your answer in the Excel file in Google drive. d) Calculate a and the partial pressures of the components for the reaction at 1 bar and the temperature you are assigned. Place your answers in the Excel file in Google drive. e) Calculate a and the partial pressures of the components for the reaction at the pressure and temperature you are assigned. Place your answers in the Excel file in Google drive f) Do your results for the above calculations agree with the prediction of LeChatelier's principle? P4. In this problem, you will calculate the degree of dissociation, a, and the partial pressure of product formed for the oxidation of sulfur dioxide under different pressure and temperature conditions. 2 SO2(g) + O2(g) + 2 SO3(g) The equilibrium constant for this reaction is 3.68 (with p = 1 bar) at 940 K. Assigned temperatures are given in an Excel file in Google drive: HW4_SO2-SO3-reaction_2022. Place your answers in this file, and hand in your calculations. a) Derive expressions for the partial pressures of each component in terms of the degree of dissociation, a, and the total pressure p. Start with 2 moles of SO2, I moles of O2, and no product. Then write the expression for the equilibrium constant. b) Determine a for p = 1.00 bar at 940 K. Then, calculate the partial pressures of all of the components. c) Calculate the equilibrium constant for the reaction the temperature you are assigned. Unless you can find a better value, use the enthalpy of the reaction calculated at 298 K. Place your answer in the Excel file in Google drive. d) Calculate a and the partial pressures of the components for the reaction at 1 bar and the temperature you are assigned. Place your answers in the Excel file in Google drive. e) Calculate a and the partial pressures of the components for the reaction at the pressure and temperature you are assigned. Place your answers in the Excel file in Google drive f) Do your results for the above calculations agree with the prediction of LeChatelier's principle p=10
T=800 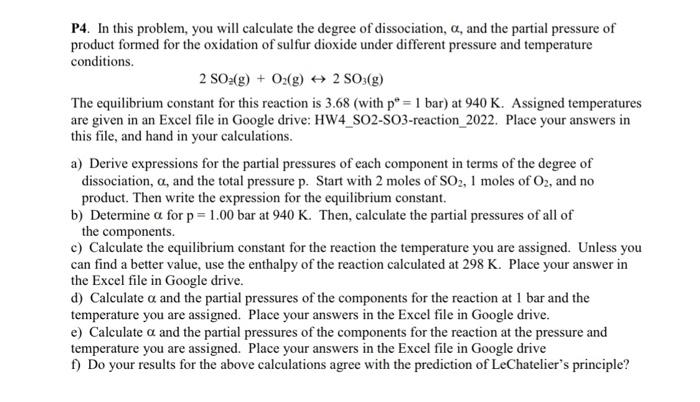

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


