Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help, please. 4. Given the following gel shown on the right, calculate the approximate molecular weight of the bands using the molecular weights
I need help, please. 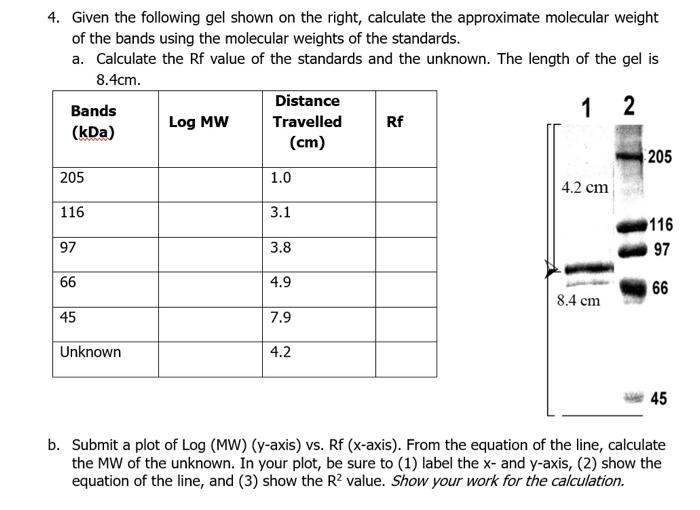
4. Given the following gel shown on the right, calculate the approximate molecular weight of the bands using the molecular weights of the standards. a. Calculate the Rf value of the standards and the unknown. The length of the gel is 8.4cm. Bands Distance 1 2 Log MW (kDa) Travelled Rf (cm) 205 205 1.0 4.2 cm 116 3.1 116 97 3.8 97 66 4.9 66 8.4 cm 45 7.9 Unknown 4.2 45 b. Submit a plot of Log (MW) (y-axis) vs. Rf (x-axis). From the equation of the line, calculate the MW of the unknown. In your plot, be sure to (1) label the x- and y-axis, (2) show the equation of the line, and (3) show the R2 value. Show your work for the calculation 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


