Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help please A. Spectrophotometric determination of max of nickel(II) ion Experimental mass of ammine-nickel complex used in solution preparation begin{tabular}{|l|l|l|} hline mass of
i need help please 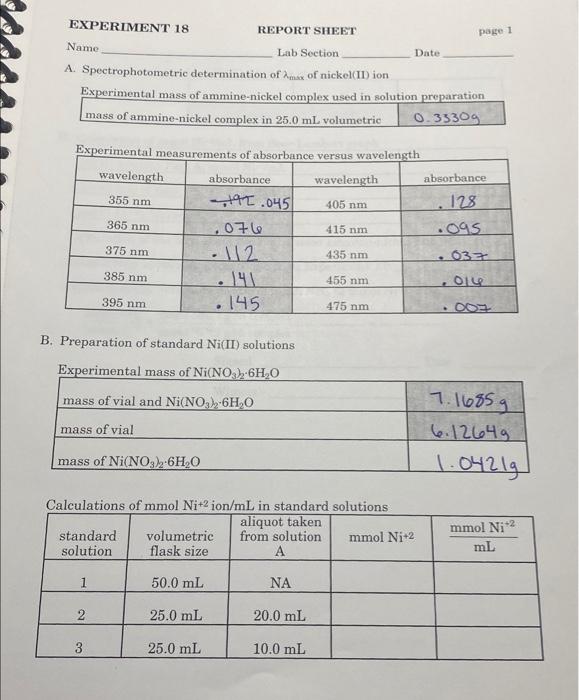
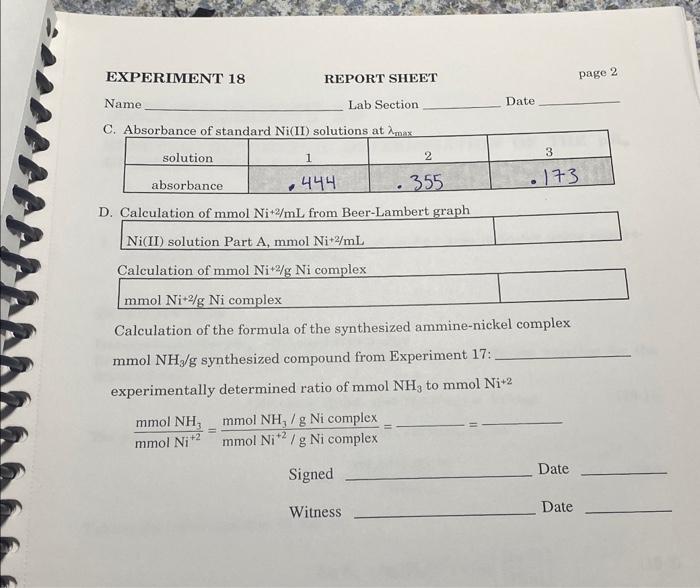
A. Spectrophotometric determination of max of nickel(II) ion Experimental mass of ammine-nickel complex used in solution preparation \begin{tabular}{|l|l|l|} \hline mass of ammine-nickel complex in 25.0mL volumetric & 0.3330g \\ \hline \end{tabular} Experimental B. Preparation of standard Ni(II) solutions EXPERIMENT 18 REPORT SHEET page 2 Name Lab Section Date D. Calculation of mmolNi+2/mL from Beer-Lambert graph Ni(II) solution Part A,mmolNi+2/mL Calculation of mmolNi+2/gNi complex mmolNi+2/gNi complex Calculation of the formula of the synthesized ammine-nickel complex mmolNH3/g synthesized compound from Experiment 17 : experimentally determined ratio of mmolNH3 to mmolNi+2 Signed Date Witness Date 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


