 I need help with 7-2 please! will upvote, Thanks
I need help with 7-2 please! will upvote, Thanks
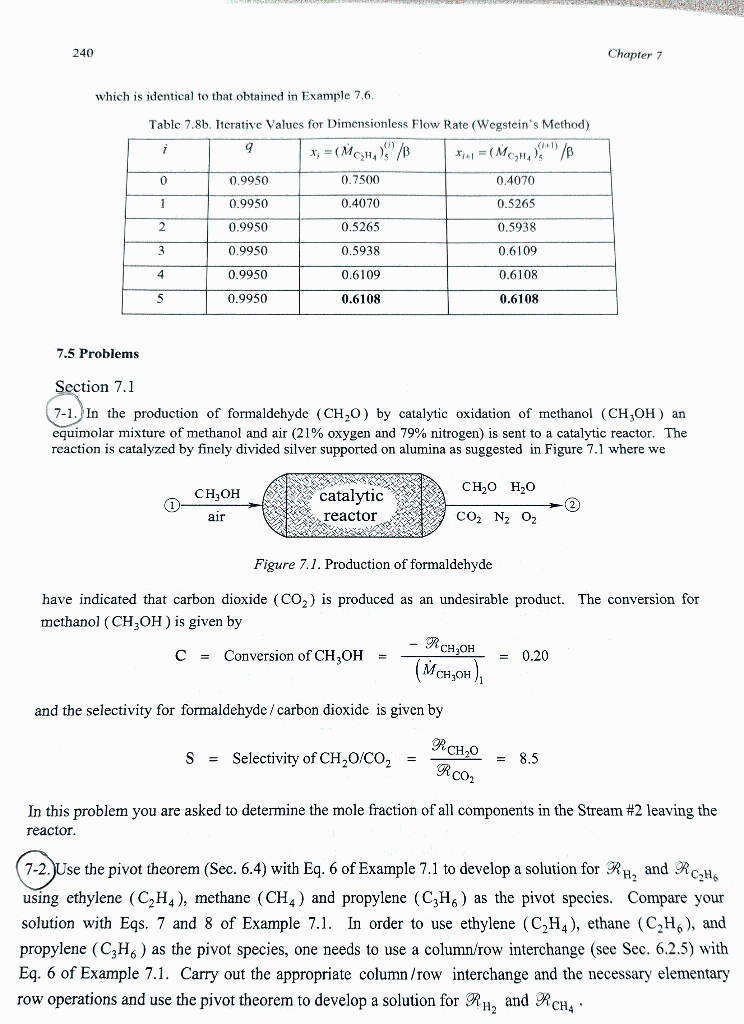
which is identical to that obtained in Example 7.6. Table 7.8b. Iterative Values for Dimensionless Flow Rate (Wegstein's Method) 7.5 Problems Section 7.1 7-1. In the production of formaldehyde (CH2O) by catalytic oxidation of methanol (CH3OH) an equimolar mixture of methanol and air ( 21% oxygen and 79% nitrogen) is sent to a catalytic reactor. The reaction is catalyzed by finely divided silver supported on alumina as suggested in Figure 7.1 where we Figure 7.1. Production of formaldehyde have indicated that carbon dioxide (CO2) is produced as an undesirable product. The conversion for methanol (CH3OH) is given by C=ConversionofCH3OH=(MCH3OH)1RCH3OH=0.20 and the selectivity for formaldehyde / carbon dioxide is given by S=SelectivityofCH2O/CO2=RCO2RCH2O=8.5 In this problem you are asked to determine the mole fraction of all components in the Stream \#2 leaving the reactor. 7-2. Use the pivot theorem (Sec. 6.4) with Eq. 6 of Example 7.1 to develop a solution for RH2 and RC2H6 using ethylene (C2H4), methane (CH4) and propylene (C3H6) as the pivot species. Compare your solution with Eqs. 7 and 8 of Example 7.1. In order to use ethylene (C2H4), ethane (C2H6), and propylene (C3H6) as the pivot species, one needs to use a column/row interchange (see Sec. 6.2.5) with Eq. 6 of Example 7.1. Carry out the appropriate column/row interchange and the necessary elementary row operations and use the pivot theorem to develop a solution for RH2 and RCH4. which is identical to that obtained in Example 7.6. Table 7.8b. Iterative Values for Dimensionless Flow Rate (Wegstein's Method) 7.5 Problems Section 7.1 7-1. In the production of formaldehyde (CH2O) by catalytic oxidation of methanol (CH3OH) an equimolar mixture of methanol and air ( 21% oxygen and 79% nitrogen) is sent to a catalytic reactor. The reaction is catalyzed by finely divided silver supported on alumina as suggested in Figure 7.1 where we Figure 7.1. Production of formaldehyde have indicated that carbon dioxide (CO2) is produced as an undesirable product. The conversion for methanol (CH3OH) is given by C=ConversionofCH3OH=(MCH3OH)1RCH3OH=0.20 and the selectivity for formaldehyde / carbon dioxide is given by S=SelectivityofCH2O/CO2=RCO2RCH2O=8.5 In this problem you are asked to determine the mole fraction of all components in the Stream \#2 leaving the reactor. 7-2. Use the pivot theorem (Sec. 6.4) with Eq. 6 of Example 7.1 to develop a solution for RH2 and RC2H6 using ethylene (C2H4), methane (CH4) and propylene (C3H6) as the pivot species. Compare your solution with Eqs. 7 and 8 of Example 7.1. In order to use ethylene (C2H4), ethane (C2H6), and propylene (C3H6) as the pivot species, one needs to use a column/row interchange (see Sec. 6.2.5) with Eq. 6 of Example 7.1. Carry out the appropriate column/row interchange and the necessary elementary row operations and use the pivot theorem to develop a solution for RH2 and RCH4
 I need help with 7-2 please! will upvote, Thanks
I need help with 7-2 please! will upvote, Thanks





