Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with a and b. Thank you! Imagine fresh water containing 0.02MCa2+ and 0.02MSO42 fills an evaporite basin lined with gypsum. Assume that
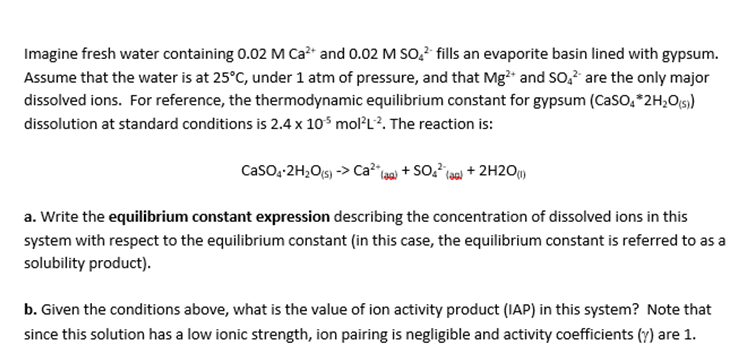
I need help with a and b. Thank you!
Imagine fresh water containing 0.02MCa2+ and 0.02MSO42 fills an evaporite basin lined with gypsum. Assume that the water is at 25C, under 1atm of pressure, and that Mg2+ and SO42 are the only major dissolved ions. For reference, the thermodynamic equilibrium constant for gypsum ( CaSO42H2O(5)) dissolution at standard conditions is 2.4105mol2L2. The reaction is: CaSO42H2O(5)>Ca2+(20)+SO4(3(2)+2H2O(1) a. Write the equilibrium constant expression describing the concentration of dissolved ions in this system with respect to the equilibrium constant (in this case, the equilibrium constant is referred to as a solubility product). b. Given the conditions above, what is the value of ion activity product (IAP) in this system? Note that since this solution has a low ionic strength, ion pairing is negligible and activity coefficients () are 1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


