Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with part e to part i Stream Table, flows in gmol/h, All at 1atm pressure Use the Flow diagram and stream table
I need help with part e to part i 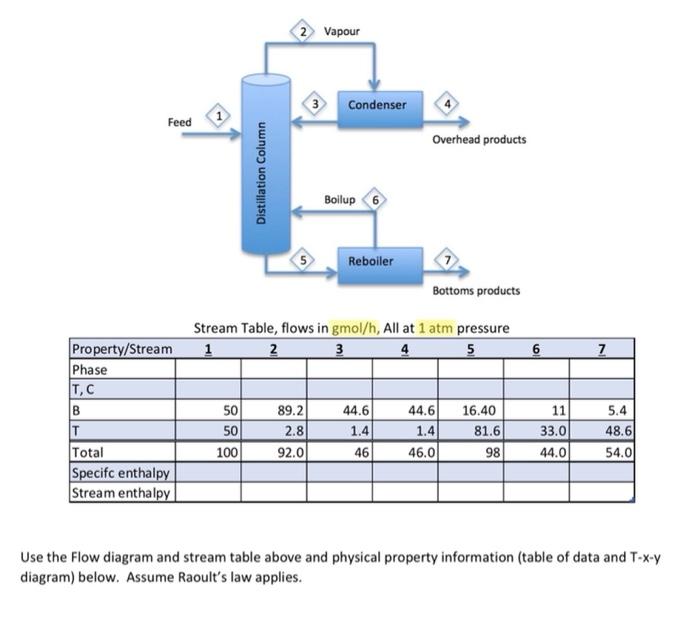

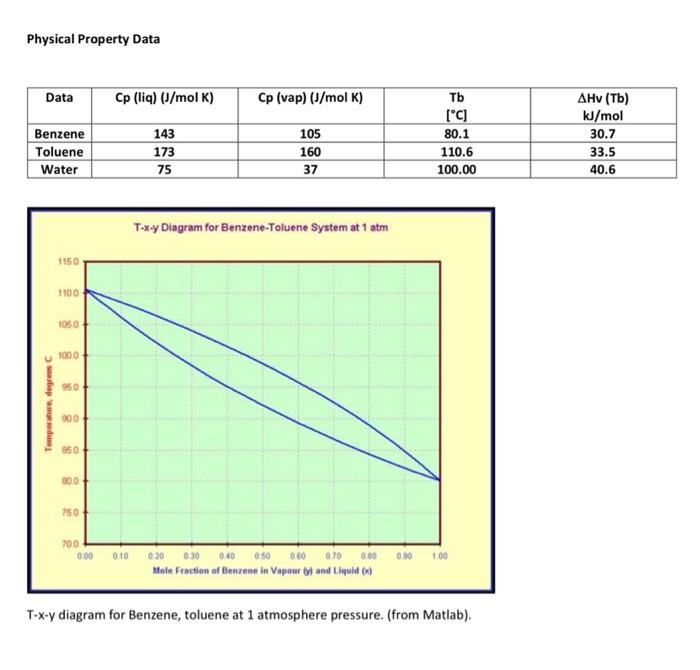
Stream Table, flows in gmol/h, All at 1atm pressure Use the Flow diagram and stream table above and physical property information (table of data and T-x-y diagram) below. Assume Raoult's law applies. a. Enter the phases of each stream except stream 1, which we will determine later. b. Provide a suitable reference state for benzene, toluene (Hint, what is the lowest temperature you are likely to see?) and water, used in the condenser and reboiler but which does not contact the process. (Hint: use the reference state for your source, often liquid water at the triple point). c. Estimate the temperatures of streams 2-7 using the Txy diagram for benzene and Toluene. Assume the streams are saturated, i.e. at either their dewpoint or bubble point. d. Determine the specific and total enthalpies of streams 2-7. e. Show the energy balance for each and calculate the heat duties of the condenser and the reboiler. f. We will solve for the condition of the feed. The column itself (without the reboiler or condenser) is adiabatic. The feed is a mix of vapour and liquid. Estimate the temperature of the feed, assuming it is half vapourized, that is, xfeed=50%. Complete an energy balance around the column and estimate the temperature of the feed and vapour fraction (x) of the feed. Set up an energy balance with 2 unknowns: the vapour fraction and the heat of vapourization of the feed. Estimate the heat of vapourization Hv as the average of the 2 components. Solve for the vapour fraction. Complete the stream table. g. Once you have estimated the vapour fraction, comment on how much error was caused by the assumptions you made (temperature, using average heat of vapourization). h. Cooling water is used to remove heat from the condenser and enters the condenser at 25 C and leaves at 35C. Calculate the flow rate of water in kg/h [ 5 points] i. Saturated steam at 5 barA pressure is used heat the reboiler and exits the reboiler as a saturated liquid. Calculate the flow rate in kg/h. [ 10 points] Physical Property Data Txy diagram for Benzene, toluene at 1 atmosphere pressure. (from Matlab) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


