Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with the last page thank you Gray 18.22 g Data, Calculations and Questions Density of Solid Cylinder Color of sample Mass of
I need help with the last page thank you 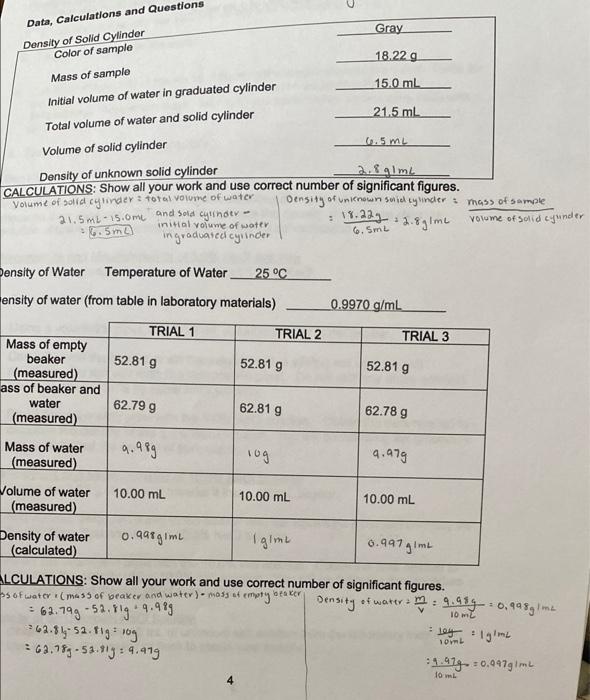
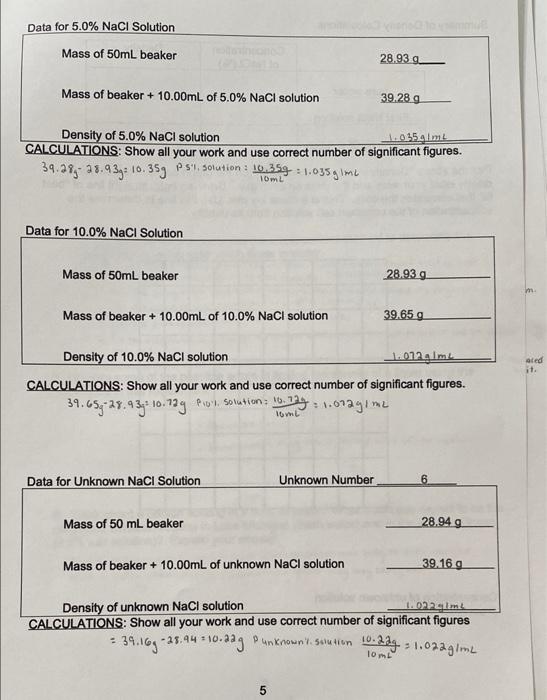
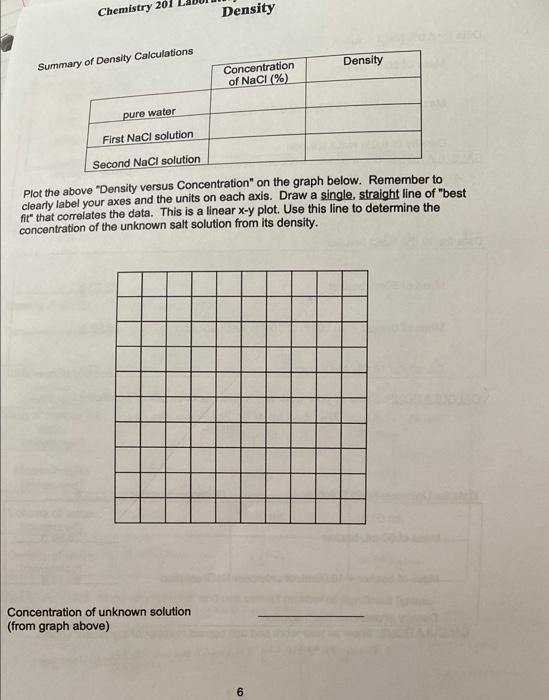
Gray 18.22 g Data, Calculations and Questions Density of Solid Cylinder Color of sample Mass of sample Initial volume of water in graduated cylinder Total volume of water and solid cylinder Volume of solid cylinder 15.0 ml 21.5 mL 60.5 mL Density of unknown solid cylinder 2. Alm CALCULATIONS: Show all your work and use correct number of significant figures. Volume of solid cylinder ? total volume of water Density of unknown su cywinder : mass of sample 21.5 mL-15.0m and sold cylinder - inical volume of water 19.224 12.8g1me volume of solid cyunder = 6.5mo ingraduated cylinder 3 2 6. SmL Density of Water Temperature of Water 25C ensity of water (from table in laboratory materials) 0.9970 g/mL TRIAL 1 TRIAL 2 TRIAL 3 52.81 g 52.81 9 52.81 g Mass of empty beaker (measured) ass of beaker and water (measured) 62.799 62.81 g 62.78 g Mass of water (measured) 9.989 log 9.979 Volume of water (measured) 10.00 mL 10.00 mL 10.00 mL Density of water (calculated) 0.9a8gime I gimt 0.997 gimL = mi ALCULATIONS: Show all your work and use correct number of significant figures. ss of water (mass of beaker and water) - Mossef emotyweater Density of water 9.914--0,999mu = 62.799-52.81g .9.989 62.81-52.81g 10g 269.789-53.193: 4.419 91.979.: 0.4479mL IO soy 1gimi OL 10 mL Data for 5.0% NaCl Solution Mass of 50mL beaker 28.93 - Mass of beaker + 10.00mL of 5.0% NaCl solution 39.28 9 Density of 5.0% NaCl solution 1.025.4 m CALCULATIONS: Show all your work and use correct number of significant figures. 39.38, 239 3:10.35g PS1, solution : 125g : 1.035 gimi 10mL Data for 10.0% NaCl Solution Mass of 50ml beaker 28.939 Mass of beaker + 10.00mL of 10.0% NaCl solution 39.65 g sed it. Density of 10.0% NaCl solution 1.012 Ime CALCULATIONS: Show all your work and use correct number of significant figures. 39.65,-28.43,- 10.739 Piol, solution: 16. 16.779 1.002gime lom Data for Unknown NaCI Solution Unknown Number 6 Mass of 50 ml beaker 28.949 Mass of beaker + 10.00mL of unknown NaCl solution 39.169 1.02.20m Density of unknown NaCl solution CALCULATIONS: Show all your work and use correct number of significant figures 34.169 -25.94 * 10.987 Punknown.l.solution 1.07agime 5 Chemistry 201 Density Summary of Density Calculations Density Concentration of NaCl (%) pure water First NaCl solution Second NaCl solution Plot the above "Density versus Concentration" on the graph below. Remember to clearly label your axes and the units on each axis. Draw a single, straight line of "best fire that correlates the data. This is a linear x-y plot. Use this line to determine the concentration of the unknown salt solution from its density. Concentration of unknown solution (from graph above) 6 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


