Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with these, thank you in advance Farzad fills a 25mL volumetric flask to the line with ethylene glycol. How should the volume
I need help with these, thank you in advance 

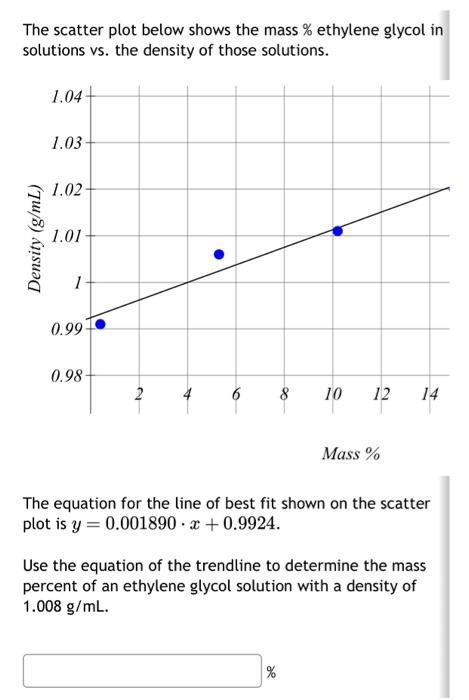
Farzad fills a 25mL volumetric flask to the line with ethylene glycol. How should the volume be reported? An ethylene glycol solution is prepared by diluting 2.000 mL of a 2.383M solution to a final volume of 50.00mL. What is the molarity of the new solution? The scatter plot below shows the mass \% ethylene glycol in solutions vs. the density of those solutions. Mass % The equation for the line of best fit shown on the scatter plot is y=0.001890x+0.9924. Use the equation of the trendline to determine the mass percent of an ethylene glycol solution with a density of 1.008g/mL. % 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


