Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with this I would appreciate the help Use the concentration in the cuvette (assay concentration) that you obtained in Question 7 to
I need help with this I would appreciate the help
Use the concentration in the cuvette (assay concentration) that you obtained in Question 7 to calculate the concentration of the unknown sample that you added to the cuvette. The concentration in the cuvette (assay concentration) is 9.87 ug/mL. Calculate the concentration of the stock solution of your unknown. (Hint use the dilution equation) V1C1=V2C2
Choices: a). 98.7 ug/mL b). 98.7 mg/mL c). 0.3 mg/mL d). 0.3 ug/mL
Extra info
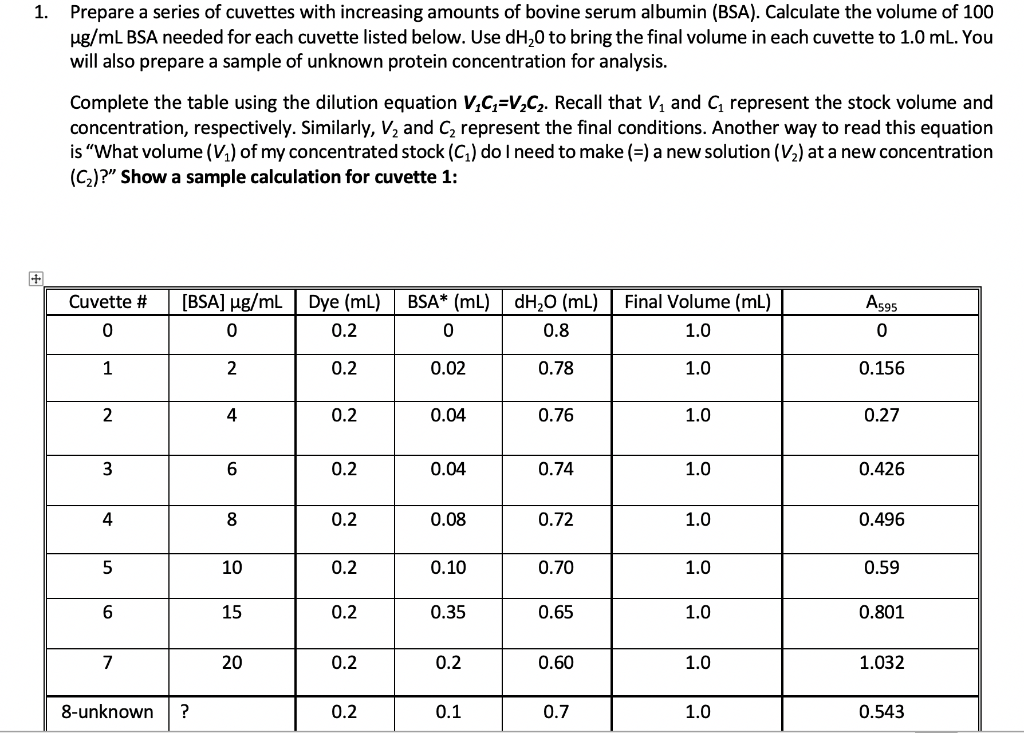
1. Prepare a series of cuvettes with increasing amounts of bovine serum albumin (BSA). Calculate the volume of 100 ug/mL BSA needed for each cuvette listed below. Use dH 0 to bring the final volume in each cuvette to 1.0 mL. You will also prepare a sample of unknown protein concentration for analysis. Complete the table using the dilution equation V.C=V_C2. Recall that V, and represent the stock volume and concentration, respectively. Similarly, V2 and C represent the final conditions. Another way to read this equation is "What volume (V) of my concentrated stock (G) do I need to make (=) a new solution (V2) at a new concentration (C2)?" Show a sample calculation for cuvette 1: Cuvette # A595 (BSA) ug/mL 0 Dye (mL) 0.2 BSA* (mL) dH20 (ml) 0 0.8 Final Volume (mL) 1.0 0 co 0 1 2 0.2 0.02 0.78 1.0 0.156 2 4 0.2 0.04 0.76 1.0 0.27 3 6 0.2 0.04 0.74 1.0 0.426 4 8 0.2 0.08 0.72 1.0 0.496 5 10 0.2 0.10 0.70 1.0 0.59 6 15 0.2 0.35 0.65 1.0 0.801 7 20 0.2 0.2 0.60 1.0 1.032 8-unknown ? 0.2 0.1 0.7 1.0 0.543
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


