Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with this question -For each of the three hydroxides, calculate at what pH would a 0.01 mol/L solution of the metal cation
I need help with this question 
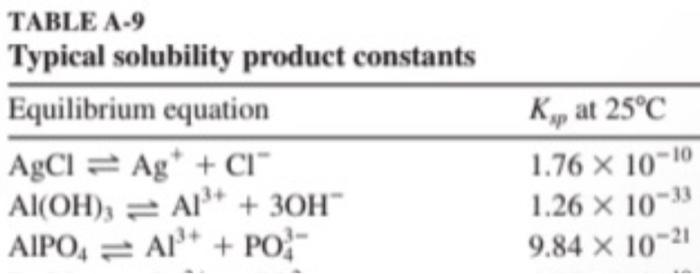
-For each of the three hydroxides, calculate at what pH would a 0.01 mol/L solution of the metal cation start to precipitate. TABLE A-9 Typical solubility product constants Equilibrium equation AgCl = Ag* + CI" Al(OH)3 = A1+ + 30H AIPO = A + PO Ky at 25C 1.76 x 10-10 1.26 X 1033 9.84 x 10-21 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


