Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with this question, please! The freezing point of benzene, CH, is 5.50C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in benzene
I need help with this question, please! 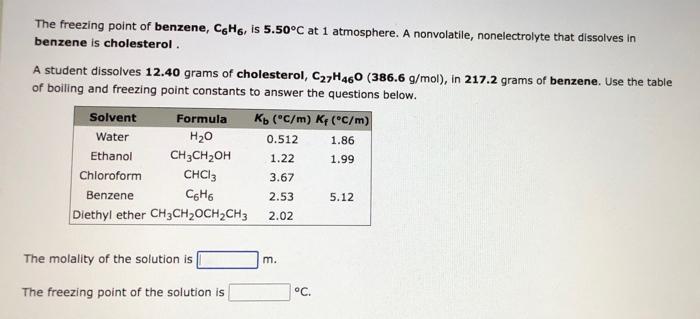
The freezing point of benzene, CH, is 5.50C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in benzene is cholesterol. A student dissolves 12.40 grams of cholesterol, C27H460 (386.6 g/mol), in 217.2 grams of benzene. Use the table of boiling and freezing point constants to answer the questions below. Solvent Formula Ko (C/m) K (C/m) Water H20 0.512 1.86 Ethanol CH3CH2OH 1.22 1.99 Chloroform CHCl3 3.67 Benzene 66 2.53 5.12 Diethyl ether CH3CH2OCH2CH3 2.02 The molality of the solution is m. The freezing point of the solution is C 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


