Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with this two part question please. 4. Consider the following gas phase esterification reaction, where butanoic acid (CaH7CO2Hth reacts with ethanol (C2H2OH)
I need help with this two part question please. 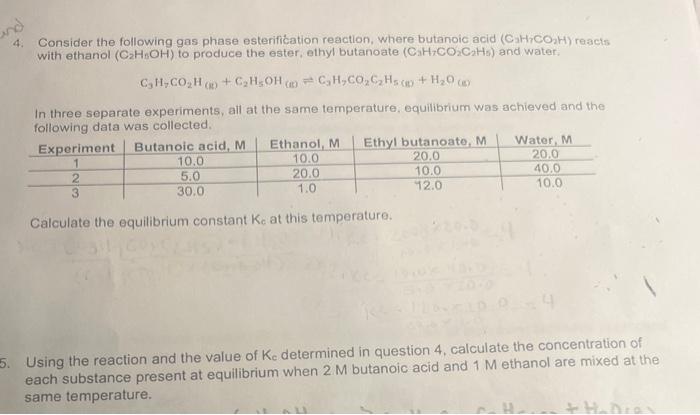
4. Consider the following gas phase esterification reaction, where butanoic acid (CaH7CO2Hth reacts with ethanol (C2H2OH) to produce the ester, ethyl butanoate (C3H7COO2C2H5) and water. C3H7CO2H(s)+C2H5OH(m)C3H7CO2C2H5sg+H2O(s) In three separate experiments, all at the same temperature, equilibrium was achieved and the followinn data was collected. Calculate the equilibrium constant Kc at this temperature. Using the reaction and the value of Kc determined in question 4 , calculate the concentration of each substance present at equilibrium when 2M butanoic acid and 1M ethanol are mixed at the same temperature 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


