I need hep with the calculating the questions 1 to 9.
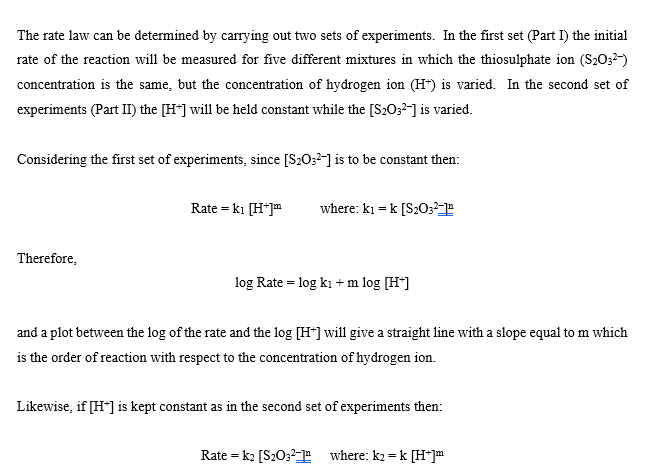
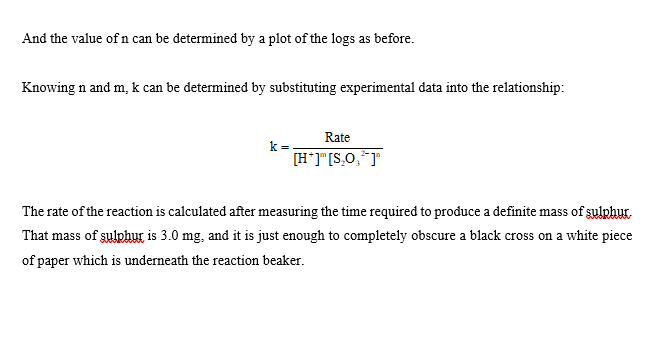
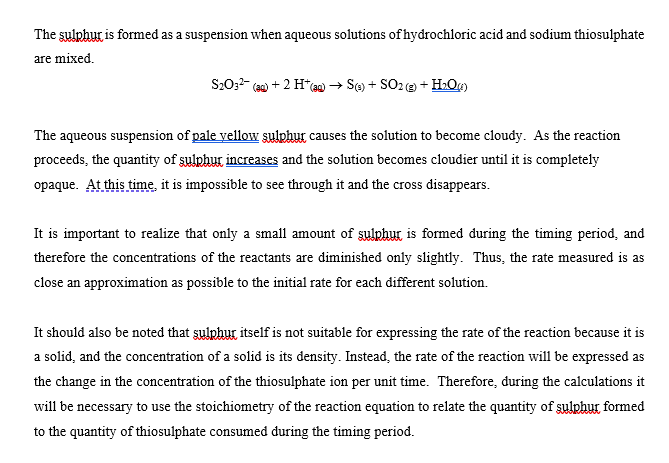
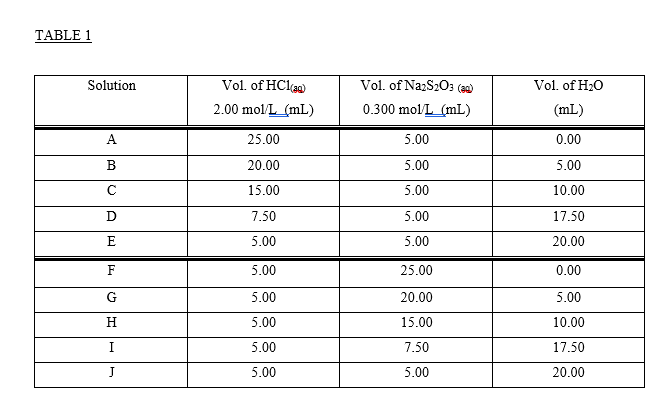


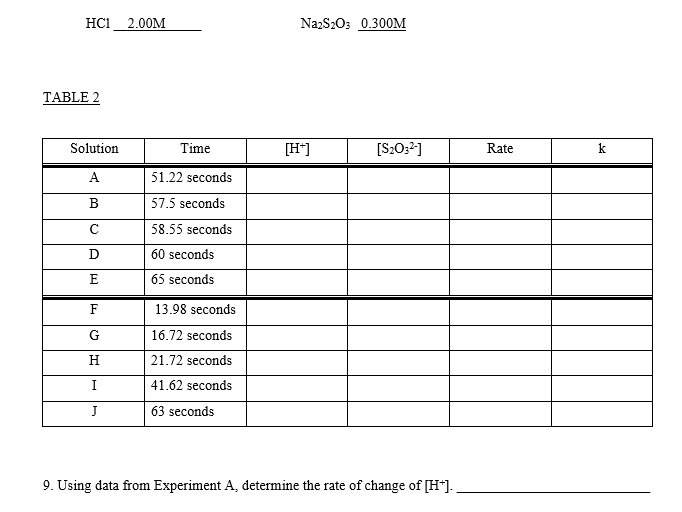
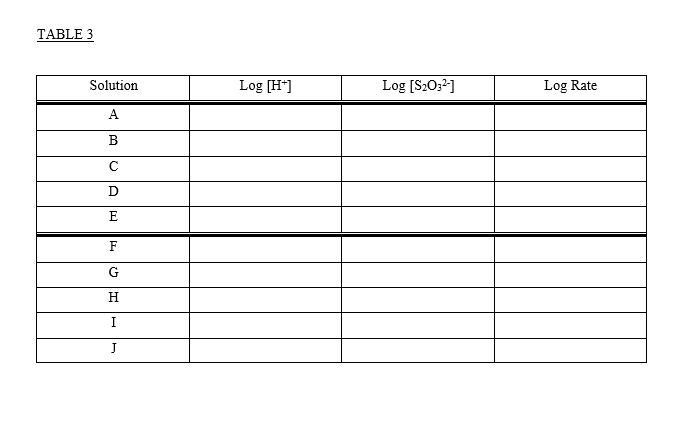
CHEMICAL KINETICS Introduction The rates at which reactions occur have social and economic implications because both time and money are important. Some scientistsoften those working in industrywant to speed up reactions so that the products can be made quickly and possibly more cheaply. Other chemists want to slow certain reactions down, particularly reactions such as the rusting of steel, the corrosion of stone work or the spoiling of food. It is therefore important to understand the factors which control the rates of reactions. One of the factors that influences the rate of reaction is the concentration of the reactants. The effect that changing the concentration of the reactants has on the rate of a reaction is always shown by the rate law for the reaction in question. Once the rate law of a reaction is known, it is possible to predict how the rate of the reaction will change when the concentration of the reactants is changed. The purpose of this experiment is to investigate how the rate of the reaction between thiosulphate ion and hydrogen ion varies when the concentrations of the reactants are changed, and to determine the rate law governing the reaction The equation for the reaction is: S2032-aa) + 2 H+(2+6) +SO2@+H:09 The rate law can be determined by carrying out two sets of experiments. In the first set (Part I) the initial rate of the reaction will be measured for five different mixtures in which the thiosulphate ion (S2032) concentration is the same, but the concentration of hydrogen ion (H+) is varied. In the second set of experiments (Part II) the [H+] will be held constant while the [S20:2-] is varied. Considering the first set of experiments, since [S20:2-] is to be constant then: Rate = ki [H+] where: ki = k [S20:-1 Therefore, log Rate = log ki + m log [H*] and a plot between the log of the rate and the log [H*] will give a straight line with a slope equal to m which is the order of reaction with respect to the concentration of hydrogen ion. Likewise, if [H*] is kept constant as in the second set of experiments then: Rate = k2 [S20:21 where: k2 = k [H+]m = And the value of n can be determined by a plot of the logs as before. Knowing n and m, k can be determined by substituting experimental data into the relationship: Rate k= [H*]"[3,0,1 The rate of the reaction is calculated after measuring the time required to produce a definite mass of sulphur- That mass of sulphur is 3.0 mg, and it is just enough to completely obscure a black cross on a white piece of paper which is underneath the reaction beaker. The sulphur is formed as a suspension when aqueous solutions of hydrochloric acid and sodium thiosulphate are mixed. S2O32- (a + 2 H+(29) +56) + SO2 +H:09 The aqueous suspension of pale yellow sulphur causes the solution to become cloudy. As the reaction proceeds, the quantity of sulphur increases and the solution becomes cloudier until it is completely opaque. At this time it is impossible to see through it and the cross disappears. It is important to realize that only a small amount of sulphur is formed during the timing period, and therefore the concentrations of the reactants are diminished only slightly. Thus, the rate measured is as close an approximation as possible to the initial rate for each different solution. It should also be noted that sulphur itself is not suitable for expressing the rate of the reaction because it is a solid, and the concentration of a solid is its density. Instead, the rate of the reaction will be expressed as the change in the concentration of the thiosulphate ion per unit time. Therefore, during the calculations it will be necessary to use the stoichiometry of the reaction equation to relate the quantity of sulphur formed to the quantity of thiosulphate consumed during the timing period. TABLE 1 Solution Vol. of H2O Vol. of HC120 2.00 mol/L (mL) Vol. of Na2S2O3 () 0.300 mol/L (mL) (mL) A 25.00 5.00 0.00 B 20.00 5.00 5.00 15.00 5.00 10.00 D 5.00 7.50 5.00 17.50 20.00 E 5.00 F 5.00 25.00 0.00 G 5.00 5.00 H 5.00 20.00 15.00 7.50 10.00 I 17.50 5.00 5.00 J 5.00 20.00 Temperature of the solutions: 23.5C Calculations: 1. Calculate the [H+] and the [S2032-] for each reacting solution based on the total volume and enter the results in Table 2. 2. Calculate the reaction rate for each run as follows: It was determined experimentally that the ink cross is no longer visible after 3.0 mg of sulphur was formed in 30.0 mL of solution). W a) Calculate the change in the moles of sulphur needed to obscure the cross. b) Use the balanced chemical equation to calculate the change in the moles of thiosulphate ion to form the moles of sulphur calculated in part a) c) Calculate the change in concentration of thiosulphate ions (in mol/L) d) Calculate the rate of the reaction in terms of the change in concentration of thiosulphate ions per second for each reacting solution and enter the results in Table 2. 3. Calculate the values for the logs of the [H+], the logs for the [S2032-), and the logs for the rate. Enter results in Table 3. 4. Plot the log Rate against log [H*] using the data from experiments A to E. Plot the log Rate against log [S2O32-] using the data from experiments F to J. Use the same scale for both graphs. Measure the slope of each graph and determine the order for each reactant. 5. Enter the values of m: and 11: 6. The rate equation: Rate = k [H+] [S2O32-19 = may be rearranged to read: Rate k= [H*]" [S, 0,"] Using the experimentally determined values for m and n (rounded off to the nearest integer), calculate k for each solution. Enter the values in Table 2. 7. Calculate the average value for k: 8. Write the overall rate law for the reaction including the calculated value of k with its proper units. HC1 2.00M Na2S203 0.300M TABLE 2 Solution Time [H] [S2032) Rate k A 51.22 seconds B 57.5 seconds C 58.55 seconds D 60 seconds 65 seconds E F G H 13.98 seconds 16.72 seconds 21.72 seconds 41.62 seconds 63 seconds I J 9. Using data from Experiment A, determine the rate of change of [H+). TABLE 3 Solution Log [H] Log [S2032] Log Rate A B MUA D 1) G H I J
















