i need solution for question 3 please
X=0.39
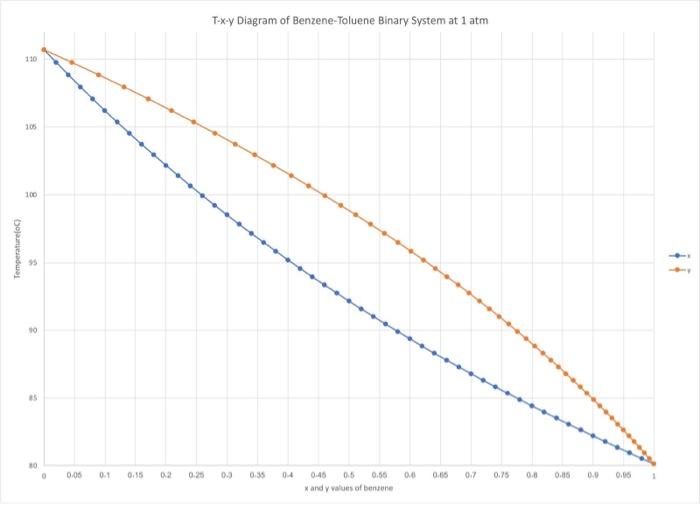
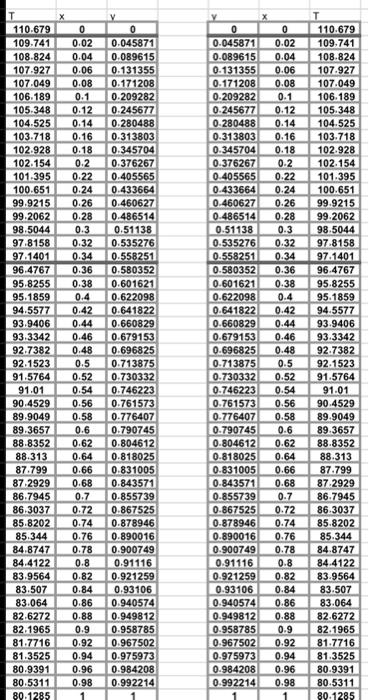
Question 1: Collect the equilibrium T - x - y data for Benzene-Toluene binary system at 1 atm pressure from an authentic source (e.g. journal article, Aspen Plus etc.). Present the data in a tabular form and provide a reference to the source of the data. 15 points) Question 2: a) Plot the temperature (vertical axis) versus x and y (horizontal axis) to obtain the T - x - y diagram for the system. [5 points) b) Ploty (vertical axis) versus x (horizontal axis) to obtain the y - x diagram for the system. 15 points) Question 3: A liquid solution containing x% benzene and the rest toluene is heated at a constant pressure of 1 atm. Here x is the last two digits of your QU ID i.e. if your ID is 20080234, then x = 34. Using the T - x - y data and the diagrams obtained in Part B. answer the following questions. a) What is the composition of the first bubble of equilibrium vapor formed? [5 points) b) If you start with 100 moles of the liquid solution, what will be the composition of the residual liquid when 50 mol% of the liquid has evaporated? Provide solution to this problem using (1) the T-x-y diagram and (ii) the yx diagram. Assume that all vapor formed is retained within the apparatus while maintaining the pressure at 1 atm and that the vapor is in equilibrium with the residual liquid. 15 points) T-x-y Diagram of Benzene-Toluene Binary System at 1 atm 110 105 100 1 Temperature) 10 15 30 0.00 0.1 0.15 02 0.25 0.3 0.35 04 0.0 0.05 0.7 0.75 0.0 0.15 0.0 0.05 0.45 05 0.56 X and values of benene 0 0.1 110.679 109.741 108.824 107.927 107.049 106.189 105,348 104.525 103.718 102.928 102.154 101.395 100.651 99.9215 99.2062 98.5044 97.8158 97.1401 96.4767 95.8255 95.1859 94.5577 93.9406 93 3342 92.7382 92.1523 91.5764 91.01 90.4529 89.9049 89.3657 88.8352 88.313 87.799 87.2929 86.7945 86 3037 85.8202 85.344 84.8747 84.4122 83.9564 83.507 83064 82.6272 82.1965 81.7716 81.3525 80.9391 80.5311 80.1285 X 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.18 0.2 0.22 0.24 0.26 0.28 0.3 0.32 0.34 0.36 0.38 0.4 0.42 0.44 0.46 0.48 0.5 0.52 0.54 0.56 0.58 0.6 0.62 0.64 0.66 0.68 0.7 0.72 0.74 0.76 0.78 0.8 0.82 0.84 0.86 0.88 0.9 0.92 0.94 0.96 0.98 1 V 0 0.045871 0.089615 0.131355 0.171208 0.209282 0.245677 0.280488 0.313803 0.345704 0.376267 0.405565 0.433664 0.460627 0.486514 0.51138 0.535276 0.558251 0.580352 0.601621 0.622098 0.641822 0.660829 0.679153 0.696825 0.713875 0.730332 0.746223 0.761573 0.776407 0.790745 0.804612 0.818025 0.831005 0.843571 0.855739 0.867525 0.878946 0.890016 0-900749 0.91116 0.921259 0.93106 0.940574 0.949812 0.958785 0.967502 0.975973 0.984208 0.992214 X 0 0 0.045871 0.02 0.089615 0.04 0.131355 0.06 0.171208 0.08 0.209282 0.245677 0.12 0.280488 0.14 0.313803 0.16 0.345704 0.18 0.376267 0.2 0.405565 0.22 0.433664 0.24 0.460627 0.26 0.486514 0.28 0.51138 0.3 0.535276 0.32 0.558251 0.34 0.580352 0.36 0.601621 0.38 0.622098 0.4 0.641822 0.42 0.660829 0.44 0.679153 0.46 0.696825 0.48 0.713875 0.5 0.730332 0.52 0.746223 0.54 0.761573 0.56 0.776407 0.58 0.790745 0.804612 0.62 0.818025 0.64 0.831005 0.66 0.843571 0.68 0.855739 0.7 0.867525 0.72 0-878946 0.74 0.890016 0.76 0.900749 0.78 0.91116 0.8 0.921259 0.82 0.93106 0.84 0.940574 0.86 0.949812 0.88 0.958785 0.9 0.967502 0.92 0.975973 0.94 0.984208 0.96 0.992214 0.98 1 110.679 109.741 108.824 107.927 107.049 106.189 105.348 104.525 103.718 102.928 102.154 101.395 100.651 99.9215 99.2062 98.5044 97.8158 97.1401 96.4767 95.8255 95.1859 94.5577 93.9406 93.3342 92.7382 92.1523 91.5764 91.01 90.4529 89.9049 89.3657 88.8352 88.313 87.799 87.2929 86.7945 86.3037 85.8202 85.344 84.8747 84.4122 83.9564 83.507 83.064 82.6272 82.1965 81.7716 81.3525 80.9391 80.5311 89.1285 0.6