Question
What is the freezing point of a solution made by dissolving 10.35 g of naphthalene (C0Hg) in 100.0 g benzene (CH?)? The normal freezing

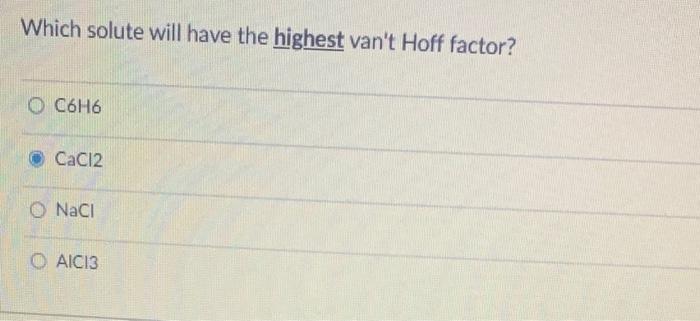
What is the freezing point of a solution made by dissolving 10.35 g of naphthalene (C0Hg) in 100.0 g benzene (CH?)? The normal freezing point of benzene is 5.5C and K f is 5.12C / m. O 0.0C O 14 06.9 O 3.4C Which solute will have the highest van't Hoff factor? O C6H6 CaCl2 O NaCl OAIC13
Step by Step Solution
3.41 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
Freezing Point of Naphthalene Solution Given Mass of naphthalene CH1035 g Mass of benzene CH1000 g F...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Mechanics of Materials
Authors: James M. Gere, Barry J. Goodno
7th edition
495438073, 978-0495438076
Students also viewed these Civil Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



